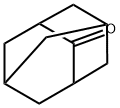
Synthesis and Spectroscopy of Adamantanone
General description
Adamantanone has been used as a probe for the size and characteristics of alcohol dehydrogenase substrate binding pockets. Adamantanone can be used for the preparation of d bisazomethines. For example, Kochetkov et al. prepared bisazomethines from D, L-camphor, 2-adamantanone and aliphatic diamine as raw materials [1].
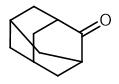
Fig. 1 The structure of 2-Adamantanone.
Physicochemical property
2-Adamantanone is white crystal, camphor flavor, and its melting point is 256-258 °C. It is soluble in methanol, ethanol, DMSO and other organic solvents. The compound must be prepared by synthesis.
Synthetic routes
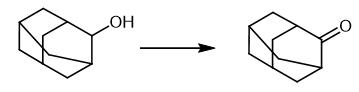
Fig. 2 The synthetic method 1 of 2-Adamantanone.
Dissolve the alcohol (0.25 mmol) in acetonitrile (3 mL). Add oxidant 1,3-dichloro-5,5-dimethylhydantoin (DCH, 0.148 g, 0.75 mmol) to the reaction mixture. Add the pre-catalyst MWCNT-{(CH2)3-CO- NH-TEMPO}n (0.075 g) to the reaction mixture. Sonic the resulting suspension (1 min.) using an ultrasonic bath. Stir the reaction mixture. Heat the reaction mixture at 50 °C for 30 minutes. Filter the reaction mixture. Add CH2Cl2 (10 mL) to the reaction mixture. Wash the organic phase with aqueous Na2S2O3 (10 %, 10 mL) and H2O (10 mL x 2). Dry the residue with Na2SO4. Remove the solvent under reduced pressure using a rotary evaporator to obtain the product [2].
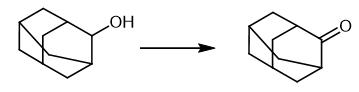
Fig. 3 The synthetic method 2 of 2-Adamantanone.
Prepare a solution of adamantane derivative (0.25 mmol) and 1,1,1-trifluoroacetone (0.9 mL) in DCM (4.1 mL) in a 20 mL vial. Keep the reaction mixture at 0°C in an ice bath. Inject the solution in a loop of 5 mL at 0°C and pump with DCM as a solvent at a flow rate of 0.33 mL.min-1, mixed in an arrowhead T-mixing piece with a solution of NaHCO3 (1.5 M) pumped at a flow rate of 1.67 mL.min-1. Mix the solution with an arrowhead T-mixing piece with a solution of oxone (0.5 M) pumped at a flow rate of 1.67 mL.min-1. Pass the mixture through a reactor (5 mL, PFA, bore tubing 1/8 x 0.093 x 5 ft) containing glass beads (425-600 μm) at 25°C using the vapourtec R4 and R2+ series. Collect the crude material in a round bottom flask. Extract the aqueous layer with DCM (3 X 10 mL). Wash the combined organic layers with brine (10 mL), dry over MgSO4. Filter and concentrate the mixture to dryness. Purify the residue by flash chromatography on silica gel (DCM/MeOH 70/30) to obtain adamantan-2-one.1H NMR (400 MHz, CDCl3, 298K, TMS): δ(ppm) = 2.55 (s, 2H), 2.12-2.06 (m, 4H), 2.05-1.96 (m, 6H), 1.93 (s, 2H). 13C NMR HSQC NMR (101 MHz, CDCl3, 298 K, TMS): δ(ppm) = 218.5, 47.0, 39.3, 36.3, 27.5 [3].
Spectroscopic research
Mass-Spectral Study
The mass spectral fragmentation of 2-adamantanone has been investigated using its specifically labelled monodeuterated derivatives (in positions 1-, 4-exo-, 4-endo-, 5- and 6-) and dideuterated derivatives (in positions 4,4- and 6,6-). The mass spectrum of 2-adamantanone is generally poor in fragments showing the molecular ion as the base peak, the characteristic m/z 117, 104, 93, 91, 80, 79, 72, 67 and 53 fragments, as well as an unusual loss of water. The mass spectra of deuterated compounds favours the assumption of hydrogen randomization prior to the fragmentation of the molecular ion. In contrast, the formation of [C4H8O]+., m/z 72 does not involve any major hydrogen scrambling [4].
Low-temperature Raman spectra of 2-adamantanone in different phases
The experimental results that show the existence of different low-temperature phases of 2-adamantanone (glassy crystal state, ordered crystal state and amorphous state in the form of molecular film) are presented. The glassy crystal state is obtained by rapid cooling of the room-temperature plastic phase. The freezing involves primarily the molecular rotational degrees of freedom while the translational order of the plastic phase is preserved. The phase transition to the ordered crystal state can be induced by very slow annealing between the room temperature and 10 K. Amorphous 2-adamantanone films were prepared by vacuum evaporation and deposition onto cold metal substrate at temperature 10 K. The obtained low-temperature structures of 2-adamantanone exhibit substantially different low-frequency Raman spectra (100-10 cm-1) when analysed by means of reduced Raman intensity I-R(omega) [5].
Enolates in 3-D
Deprotonation of 2-adamantanone in the gas phase affords the corresponding beta-enolate anion. This ion was independently prepared by the fluoride-induced desilylation of 4-trimethylsilyl-2-adamantanone, and its reactivity and thermodynamic properties were measured (Delta H-acid degrees = 394.7 +/- 1.4, EA = 16.8 +/- 1.6, and BDE = 97.9 +/- 2.1 kcal mol-1). Density functional theory calculations with B3LYP and M06-2X, and G3 energies arc also reported. The computed relative stabilities of the conjugate bases of I are as follows: beta > gamma > alpha > delta. An attempt to prepare the gamma-anion, however, resulted in the formation of its ring-opened isomer (i.e., deprotonated 7-methylenebicyclo[3.3.1]nonan-2-one) [6].
References
[1] Kochetkov V G, Burmistrov V V, D’yachenko V S, et al. Synthesis and Study of Framework Azomethine Compounds as Ingredients of Rubber Stocks[J]. Russian Journal of Applied Chemistry, 2020, 93(6): 801-806.
[2] Gambarotti C, Bj?rsvik H R. Amino‐TEMPO Grafted on Magnetic Multi‐Walled Nanotubes: An Efficient and Recyclable Heterogeneous Oxidation Catalyst[J]. European Journal of Organic Chemistry, 2019, 2019(6): 1405-1412.
[3] Lesieur M, Battilocchio C, Labes R, et al. Direct Oxidation of Csp3? H bonds using in Situ Generated Trifluoromethylated Dioxirane in Flow[J]. Chemistry–A European Journal, 2019, 25(5): 1203-1207.
[4] Srzi? D, Vinkovi? V, Mlinari?‐Majerski K. Mass spectral study of 2‐adamantanone[J]. Rapid Communications in Mass Spectrometry, 1990, 4(12): 500-502.
[5] Bistricic L, Baranovi? G, Volovsek V. Low-temperature Raman spectra of 2-adamantanone in different phases[J]. Journal of molecular structure, 1999, 482: 661-664.
[6] Meyer M M, Kass S R. Enolates in 3-D: An Experimental and Computational Study of Deprotonated 2-Adamantanone[J]. The Journal of Organic Chemistry, 2010, 75(12): 4274-4279.
See also
Lastest Price from 2-Adamantanone manufacturers

US $0.00/kg2025-09-28
- CAS:
- 700-58-3
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 1
US $1.10/g2025-09-17
- CAS:
- 700-58-3
- Min. Order:
- 1g
- Purity:
- 99.9%
- Supply Ability:
- 100 Tons Min