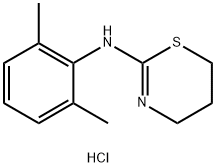
Synthesis and pharmacokinetics of xylazine hydrochloride
General description
Xylazine hydrochloride is a non-narcotic drug synthesized in 1962 by Bayer (Leverkusen, Germany), used as a sedative, analgesic, and muscle relaxant in animals[1]. According to the Food and Drug Administration (FDA), xylazine hydrochloride is used exclusively in veterinary medicine, marketed as Rompun?, Anased?, Sedazine?, and Chanazine?. It is approved for use in dogs, cats, horses, fallow deer (dama dama), mule deer (odocoileus hemionus), sika deer (cervus nippon), white-tailed deer (odocoileus virginianus), and elk (cervus canadensis). Its chemical structure closely resembles the phenothiazines, tricyclic antidepressants, and clonidine . Xylazine hydrochloride is a potent α2-adrenergic agonist that mediates via stimulation of central α2-receptors[2-3]. The α2 stimulation decreases the release of norepinephrine and dopamine in the central nervous system resulting in sedation, muscle relaxation, and decreased perception of painful stimuli. Its actions may also involve cholinergic, serotonergic, dopaminergic, α-1-adrenergic, histaminergic, or opiate mechanisms. Acknowledged side effects in animals include transient hypertension, hypotension, and respiratory depression[4].
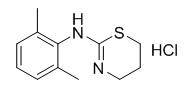
Fig. 1 The structure of xylazine hydrochloride.
Synthesis

Fig. 2 The synthetic route of xylazine hydrochloride[5].
2, 6-Xylidine (50.0g, 0.41 mol) was taken in a reaction flask and water (500ml_) was added to it at ambient temperature. Triethyl amine (45.5g, 0.45mol) was then added to it, followed by addition of carbon disulphide (32.5g, 0.43mol) at about 15 0C. The temperature was maintained for 6 hours. 3-Amino-1- propanol (30.8g, 0.41 mol) was then added over a period of 20 minutes to the reaction mixture at ambient temperature. The reaction mixture was then heated at about 80 0C for about 6 hours. The reaction mixture was then cooled to ambient temperature and concentrated hydrochloric acid (100mL) was added. The temperature was slowly raised to about 95 0C and was maintained for additional 3 hours. The reaction mixture was then cooled to about 20 0C and pH was adjusted to 9.5 to 10.5 by careful addition of sodium hydroxide solution (20%) over a period of about 35 minutes at about 20 0C. The resulting mixture was stirred at the same temperature for 1 hour. The mixture was then filtered and solid so obtained was washed with water and sucked well. It was again washed with acetone (100mL) to obtain the desired pure compound xylazine hydrochloride with purity of more than 99.5% by High Performance Liquid Chromatography (HPLC).
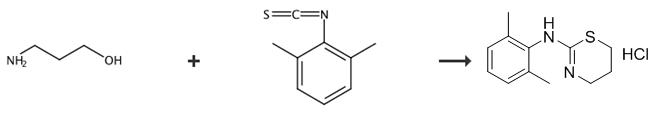
Fig. 3 The synthetic route of xylazine hydrochloride[6].
745 grams (9.8 mol) of carbon disulfide and 1230 milliliters (ml) of concentrated ammonium hydroxide was added to a 5 liter, 3-neck flask fitted with a mechanical stirrer, teflon clad thermocouple, and an additional funnel. The contents ofthe flask were cooled to 0° C. by use of an ice bath. Then with rapid stirring, 1,008 grams of 2,6-dimethylaniline was added drop wise to the contents of the flask. The temperature was maintained below 10° C. by controlling the rate of addition. It is preferable to maintain the temperature in the range of about 10° C. Ethyl chloroformate (890.8 grams; 8.21 moles) was added dropwise to the stirred slurry at a rate such that the temperature did not rise above 40° C.; a cooling bath was used to help control the temperature. The addition funnel was replaced by a condenser, and the reaction mixture was heated to between 60° and 70° C. and maintained at this temperature for I hour. The reaction mixture was cooled to ambient temperature and then I liter of water was added. The layers were separated, and the bottom layer (2,6-dimethylphenylisothiocyanate) was washed with 200 ml ofwater and then dryed over anhydrous sodium sulfate. Distillation under vaccuum atb125°, gaving 949 grams (55.2% yield on total theoretical basis by weight) of xylazine hydrochloride.
Application
Xylazine hydrochloride ((/V-2,6-dimethylphenyl)-5,6-dihydro- 4/-/-1,3-thiazin-2-amine) is used in veterinary medicine as an analgesic, muscle relaxant and sedative. It is an agonist at the a2 class of adrenergic receptor[7-8]. Xylazine hydrochloride is not a controlled substance; it is marketed as a veterinary drug and used as a sedative, analgesic and muscle relaxant. In humans, it could cause central nervous system depression, respiratory depression, bradycardia, hypotension, and even death. There have been publications of 43 cases of xylazine intoxication in humans, in which 21 (49%) were non-fatal scenarios and 22 (51%) resulted in fatalities. Most of the non-fatal cases required medical intervention. Over recent years xylazine hydrochloride has emerged as an adulterant in recreational drugs, such as heroin or speedball (a cocaine and heroin mixture). From the 43 reported cases, 17 (40%) were associated with the use of xylazine hydrochloride as an adulterant of drugs of abuse. Its chronic use is reported to be associated with physical deterioration and skin ulceration[9]. Literature shows some similar pharmacologic effects between xylazine hydrochloride and heroin in humans. These similar pharmacologic effects may create synergistic toxic effects in humans. Therefore, fatalities among drug users may increase due to the use of xylazine hydrochloride as an adulterant. Xylazine hydrochloride alone has proven harmful to humans and even more when it is combined with drugs of abuse[10].
Pharmacokinetic parameters
Xylazine hydrochloride pharmacokinetic parameters are well-established in different animal species, but not in humans. Briefly described, xylazine hydrochloride is absorbed, metabolized and eliminated extremely rapid[11]. After intravenous administration in animals, xylazine hydrochloride rapidly distributes, concentrating in the kidney and the central nervous system. The duration of effects begins within a few minutes and last up to 4 h. The pharmacokinetics of xylazine hydrochloride have been studied in the dog, sheep, horse, and cattle. Pharmacokinetic parameters do not vary greatly between species following intravenous administration. In all four species after intravenous administration, the distribution half-life (t1/2α) was very short (1.21–5.97 min) and the elimination half-life (t1/2β) varied from 23.11 to 49.51 min[12]. These values indicate that the xylazine hydrochloride concentration would decrease to an undetectable level within a few hours. Xylazine hydrochloride has a large volume of distribution (1.9–2.5 for horse, cattle, sheep and dog) suggesting that xylazine hydrochloride, as expected for the lipophilic nature of the compound, diffuses extensively. Xylazine hydrochloride bioavailability (intramuscular to intravenous) ranges from 52 to 90% in the dog, 17 to 73% in sheep, and 40 to 48% in the horse. Peak plasma concentrations are reached in 12–14 min in all species. The LD50 for dogs and horses
You may like
See also
Lastest Price from Xylazine Hydrochloride manufacturers

US $0.00/kg2025-03-18
- CAS:
- 23076-35-9
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 10T

US $30.00-20.00/kg2024-06-11
- CAS:
- 23076-35-9
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 100 tons