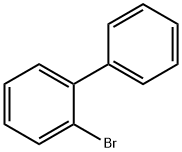
Synthesis and Application of 2-Bromobiphenyl
General description
2-Bromobiphenyl is an important intermediate, which can be used to synthesize 2,2'-dibromo-9,9'-spirobifluorene, 3-bromo-9-(9-phenylfluoren-9-yl)carbazole and phenanthrene compounds.
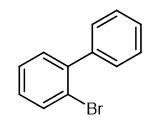
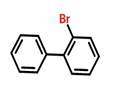
Fig. 1 The structure of 2-Bromobiphenyl.
Physicochemical property
2-Bromobiphenyl has a melting point of 1.5-2 °C, so it is a colorless liquid at room temperature. It boils at 297-298 °C. Its density at 25℃ is 1.352 g/mL. In general, 2-bromobiphenyls are stable, but they are not compatible with oxidants.
Synthetic routes
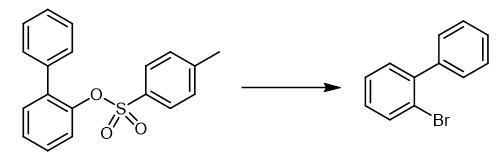
Fig. 2 The synthetic method 1 of 2-Bromobiphenyl.
Charge a pre-dried (2·10-2 mbar, heat-gun) Schlenk tube with activated zinc dust (523 mg, 8.00 mmol, 4.0 equivalents). Dry the tube under vacuum (2·10-2 mbar). Stir the mixture with a heat-gun. Add 1,2-dibromoethane (98%, 35.2 μL, 75.1 mg, 400 μmol, 0.2 equivalent) to the mixture. Rinse the walls with dry DMF (2 mL) (heat and gas evolution is observed). Stir the reaction mixture at 60 °C for 20 minutes. Cool down the reaction mixture to room temperature for five minutes in a water bath. Add NiCl2 (22.0 mg, 100 μmol, 5 mol%) and IPr-MeDAD (80.9 mg, 200 μmol, 10 mol%) to the reaction mixture. Rinse the walls with dry DMF (2 mL). Stir the brownish-green suspension for 30 minutes at room temperature. Add 1-naphthyl tosylate (597 mg) to the reaction mixture. Rinse the walls with DMF (2 mL). Stir the reaction mixture for 20 hours at room temperature. After catalytic zincation, cool the reaction mixture to 0 °C. Add N-bromosuccinimide (1.42 g, 8.00 mmol, 4.0 equivalents) to the reaction mixture. Stir the mixture for ten minutes at 0 °C. Add a saturated aqueous NH4Cl (5 mL/mmol) and sodium sulfite (1.20 g) to the reaction mixture. Stir the mixture until most of the brown color had faded. Add Et2O (10 mL) to the mixture. Filter the suspension. Separate the organic phase. Wash the organic phase with a saturated aqueous NH4Cl (2×20 mL) and NaCl (20 mL). Dry the organic phase (MgSO4). Filter the organic phase. Remove the solvent under reduced pressure (40 °C, 700 mbar). Purify the product by column chromatography (pentane). 1H NMR (400 MHz, CDCl3): δ 7.20 (ddd, 3J = 8.0, 6.8, 4J = 2.3 Hz, 1H), 7.31-7.46 (m, 7H), 7.65-7.69 (m, 1H). 13C NMR (101 MHz, CDCl3): δ 22.8, 127.5, 127.7, 128.1, 128.8, 129.5, 131.4, 133.2, 141.3, 142.7 [1].
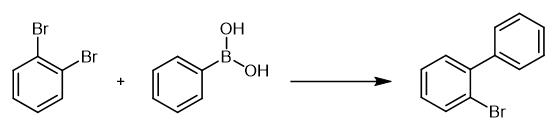
Fig. 3 The synthetic method 2 of 2-Bromobiphenyl.
Carry out all catalytic reactions in reaction vessels open to the air. Charge a round-bottom flask with the newly purchased or freshly recrystallized aryl boronic acid (2.0 mmol), 1,2-dibromobenzene, powdered K3PO4 (2.2 mmol) and toluene (2.5 mL) of technical quality. Stir the mixture vigorously. Heat the mixture to 80 °C for 10 minutes. Add (0.2 mol%) of catalyst by syringe as a 2.5 ml of toluene solution to the mixture. Take the samples periodically from the reaction mixture. Quench the reaction with water. Extract the mixture with ethyl acetate. Analyze the reaction by GC-MS. At the end of catalytic reaction, cool the reaction mixture to room temperature. Quench the mixture with water (adjusted to an appropriate pH when biaryls with acidic or basic groups has to be extracted). Extract the mixture with ethyl acetate (3×40 mL). Dry the combined extracts (MgSO4). Evaporate the combined extracts to dryness. Purify the crude material by flash chromatography on silica gel [2].
Application
For preparation of trispirocyclic hydrocarbon
A novel trispirocyclic hydrocarbon having three 9-fluorene moieties around the core of truxene (5) was prepared readily via coupling of truxenone with 2-bromobiphenyl; 5 was a high melting (> 500 degrees C) solid. For the application of 5 to an effective hole transport material (HTM) in the OLED, a triphenylamine derivative carrying six diphenylamino groups at the 2- and 7-positions of each 9-fluorene moiety (6) was designed in order to get high thermal stability as an improved material of the TPD type HTM. The synthesis of 6 was easily achieved using 4,4'-bis(diphenylamino)-2-bromobiphenyl (9). The trispirocyclic 6 was found to show a glass transition temperature as high as 170 degrees C. It effects the formation of its stable cation radical upon electrooxidation in solution, and amorphous thin films in solid. A multi-layered EL device for 6 as an HTM using Alq(3) as an electron transporting emitter showed good EL characteristics such as the maximum luminance of 37 000 cd m(-2) at 14 V. Thus, the hexakis( diphenylamino) substituted trispirocycle 6 (TX-F6S) can be used as an efficient and thermally stable HTM in OLEDs [3].
For preparation of poly(Alkylene Biphenyl Butyltrimethyl Ammonium) (ABBA)
Narducci et al. report the synthesis of the new ionomer poly(alkylene biphenyl butyltrimethyl ammonium) (ABBA) with a backbone devoid of alkaline-labile C-O-C bonds and with quaternary ammonium groups grafted on long side chains. The ionomer was achieved by metalation reaction with n-butyllithium of 2-bromobiphenyl, followed by the introduction of the long chain with 1,4-dibromobutane. The reaction steps were followed by H-1-NMR spectroscopy showing the characteristic signals of the Br-butyl chain and indicating the complete functionalization of the biphenyl moiety. The precursor was polycondensed with 1,1,1-trifluoroacetone and then quaternized using trimethylamine (TMA). After the acid catalyzed polycondensation, the stoichiometric ratio between the precursors was respected. The quaternization with TMA gave a final degree of amination of 0.83 in agreement with the thermogravimetric analysis and with the ion exchange capacity of 2.5 meq/g determined by acid-base titration. The new ionomer blended with poly(vinylalcohol) (PVA) or poly(vinylidene difluoride) (PVDF) was also characterized by water uptake (WU) and ionic conductivity measurements. The higher water uptake and ionic conductivity observed with the PVDF blend might be related to a better nanophase separation [4].
References
[1] Klein P, Lechner V D, Schimmel T, et al. Generation of organozinc reagents by nickel diazadiene complex catalyzed zinc insertion into aryl sulfonates[J]. Chemistry–A European Journal, 2020, 26(1): 176-180.
[2] Bolliger J L, Frech C M. Dichloro‐Bis (aminophosphine) Complexes of Palladium: Highly Convenient, Reliable and Extremely Active Suzuki–Miyaura Catalysts with Excellent Functional Group Tolerance[J]. Chemistry–A European Journal, 2010, 16(13): 4075-4081.
[3] Kimura M, Kuwano S, Sawaki Y, et al. New 9-fluorene-type trispirocyclic compounds for thermally stable hole transport materials in OLEDs[J]. Journal of Materials Chemistry, 2005, 15(24): 2393-2398.
[4] Narducci R, Becerra-Arciniegas R A, Pasquini L, et al. Anion-Conducting Polymer Electrolyte without Ether Linkages and with Ionic Groups Grafted on Long Side Chains: Poly (Alkylene Biphenyl Butyltrimethyl Ammonium)(ABBA)[J]. Membranes, 2022, 12(3): 337.
See also
Lastest Price from 2-Bromobiphenyl manufacturers

US $120.00/kg2025-04-21
- CAS:
- 2052-07-5
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 1000000
US $0.00/GK2025-04-21
- CAS:
- 2052-07-5
- Min. Order:
- 10g
- Purity:
- 99.50%
- Supply Ability:
- 10TONS