Synthesis and Application of 3-Amino-1,2-propanediol
General description
3-Amino-1, 2-propanediol is an important intermediate of iohexol and iodiferol, which are representative nonionic contrast agents. It is also a pesticide intermediate.
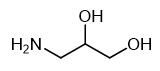
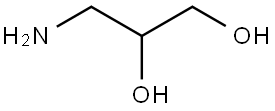
Fig. 1 The structure of 3-Amino-1,2-propanediol.
Physicochemical property
3-Amino-1,2-propanediol is a very viscous transparent colorless to pale yellow liquid with a melting point of 55-57 °C and a boiling point of 264-265 °C. The density at 25 °C is 1.175 g/mL. 3-Amino-1,2-propanediol is soluble in water.
Synthetic routes

Fig. 2 The synthetic method 1 of 3-Amino-1,2-propanediol.
The reagents were injected at flow rates of: 1-CPD (conc. 99% in water, 3.0 ml/min), NaOH (conc. 10%, 11.6 ml/min) and NH3 (conc. 25%, 56.4 ml/min). This gave a residence time of 2.87 minutes for the glycidol formation in the first and second chambers and 21.1 minutes for the aminolysis in the fourth to tenth chambers, giving a total residence time of 23.98 minutes. The temperature was held at about 42 °C in the first and second chambers and about 18 °C in the fourth to tenth chambers. In the Teflon tubing collection part the temperature was about 22 °C (ambient temperature). Samples were collected after 50 minutes from the starting point. The yield was 67.4 area% APD based on the amount of 1-CPD used. 3-aminopropane-1,2-diol or 1-aminopropane-2,3-diol, APD [1].
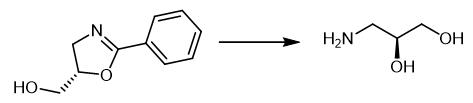
Fig. 3 The synthetic method 2 of 3-Amino-1,2-propanediol.
Add NaBH4 (16.0 mg, 0.43 mmol, 1.2 equivalents) to a solution of (2-phenyl-4,5-dihydrooxazol-5-yl)methanol (64.0 mg) in dry THF (3.60 ml). Add a solution of I2 (91.0 mg, 0.36 mmol, 1.00 equivalent) in dry THF (5.00 ml) to the mixture over 10 minutes at 0°C. Stir the mixture continuously for 16 hours at room temperature. Add aqueous NaHCO3 solution (5 mL) to the mixture. Separate the phases. Extract the aqueous phase with EtOAc (2×5 ml). Concentrate the aqueous phase. Dissolve the reaction mixture in MeOH of (3.60 ml). Add concentrated HCl (88.0 μl, 3.60 mmol, 10.0 equivalents) dropwise to the mixture. Stir the reaction mixture overnight at room temperature. Concentrate the reaction mixture. Dissolve the reaction mixture again in MeCN (3.00 ml). Add para-toluene sulfonic acid chloride (61.0 μl) to the mixture. Stir the reaction mixture for more 5 hours. Concentrate the reaction mixture. Purify the product by column chromatography (60:36:4 cyclohexane/EtOAc/MeOH). 13C NMR (150 MHz, chloroform-d) δ 142.1, 137.3, 133.7, 128.5, 69.3, 64.4, 47.7, 21.4. IR (ATR): ν (cm-1) = 3117, 2840, 2524, 1954, 1624, 1485, 1263, 845, 642. The. [α]D20 [α]23D:-17.4 (c 1.0 in CHCl3). HRMS (ESI): calculated for C10H15NO4S+ (M+H)+: m/z = 246.07341 found 246.07385. (Dev.: 0.44 mu; 1.22 ppm) [2].
Application
For the preparation of ceramide analogs
Ceramides are spingolipid compounds that are very attractive as active components in both the pharmaceutical and the cosmetic industries. In this study, the synthesis of ceramide analogs, the so-called pseudo-ceramides, was carried out using for the first time a two-step continuous enzymatic process with immobilized Candida antarctica lipase B (Novozym?435) in a packed-bed bioreactor. The first step involved the selective N-acylation of 3-amino-1,2-propanediol using stearic acid as the first acyl donor (i). This was followed by the selective O-acylation of the N-stearyl 3-amino-1,2-propanediol synthesized in the first step, with myristic acid as the second acyl donor, to produce a N,O-diacyl 3-amino-1,2-propanediol-type pseudo-ceramide, namely 1-O-myristyl,3-N-stearyl 3-amino-1,2-propanediol (ii). The process was first optimized by evaluating the influences of three factors: feed flow rate, quantity of biocatalyst and substrate concentration. Under optimal conditions an amide synthesis yield of 92% and a satisfying production rate of almost 3.15 mmol h-1 gbiocatalyst-1 (1128 mg h-1 gbiocatalyst?1) were obtained. The second step, N-acyl 3-amino-1,2-propanediol O-acylation, was similarly optimized and in addition the effect of the substrate molar ratio was studied. Thus, an optimal pseudo-ceramide synthesis yield of 54% and a production rate of 0.46 mmol h-1 gbiocatalyst-1 (261 mg h-1 gbiocatalyst-1) were reached at a 1:3 ratio of amide to fatty acid. In addition, it was demonstrated that this two-step process has great potential for the production of N,O-diacyl 3-amino-1,2-propanediol-type pseudo-ceramides on an industrial scale. It was shown in particular that Novozym?435 could be used for more than 3 weeks without a drop in the yield during the first step of 3-amino-1,2-propanediol N-acylation, proving that this biocatalyst is very stable under these operational conditions. This factor would greatly reduce the need for biocatalyst replacement and significantly lower the associated cost [3].
For the preparation of a resin with high selective adsorption of Au(III)
In Sun’s paper, a novel chelating resin with high adsorption selectivity for Au (III), polystyrene-supported 3-amino-1,2-propanediol (PS-APD), was prepared simply by the reaction of chloromethylated polystyrene with 3-amino-1,2-propanediol. Its structure was characterized by infrared spectroscopy (IR), scanning electron microscope (SEM) and porous structure analysis. The adsorption capabilities of PS-APD for Pb(II), Hg(II), Cu(II), Ni(II) and Au(III) ions were investigated. The results suggested that PS-APD resin possessed much better adsorption capability for Au(III) than for other metal ions. A comparison of the kinetic models on the overall adsorption rate showed that adsorption system was best described by the pseudo second-order kinetics. The adsorption equilibrium data fitted best with the Langmuir isotherm and thermodynamic parameters including Delta G. Delta H and Delta S were calculated. The adsorption mechanism of PS-APD for Au(III) was confirmed by SEM. IR and X-ray photoelectron spectroscopy (XPS), which showed that reclox reaction occurred between 3-amino-1,2-propanediol group and Au(III) ions. Adsorption selectivity experiments indicated that PS-APD resin possessed excellent adsorption property to Au(III) ions, offering potential applications in recovery of Au(III) from multi-ionic aqueous systems [4].
References
[1] Abazid A H, Hollwedel T N, Nachtsheim B J. Stereoselective oxidative cyclization of N-allyl benzamides to oxaz (ol) ines[J]. Organic Letters, 2021, 23(13): 5076-5080.
[2] Bolliger J L, Frech C M. Dichloro‐Bis (aminophosphine) Complexes of Palladium: Highly Convenient, Reliable and Extremely Active Suzuki–Miyaura Catalysts with Excellent Functional Group Tolerance[J]. Chemistry–A European Journal, 2010, 16(13): 4075-4081.
[3] Le Joubioux F, Bridiau N, Sanekli M, et al. Continuous lipase-catalyzed production of pseudo-ceramides in a packed-bed bioreactor[J]. Journal of Molecular Catalysis B: Enzymatic, 2014, 109: 143-153.
[4] Changmei S, Guanghua Z, Chunhua W, et al. A resin with high adsorption selectivity for Au (III): Prepara
See also
Lastest Price from 3-Amino-1,2-propanediol manufacturers

US $0.00/kg2025-04-22
- CAS:
- 616-30-8
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 1000kg

US $0.00/Kg/Drum2025-04-21
- CAS:
- 616-30-8
- Min. Order:
- 1KG
- Purity:
- 99.7%min
- Supply Ability:
- 20000kg