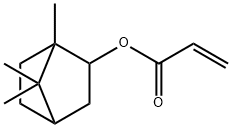
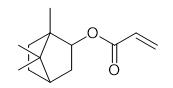
Synthesis and Hazard of Isobornyl acrylate
Isobornyl acrylate has a unique bridge ring structure, is a kind of hardness and flexibility in the integration of the excellent functional materials, such as viscosity significantly lower than the corresponding methyl ester, in copolymer and homopolymer shows good gloss, hardness, scrub resistance, medium resistance and weatherability, and hygroscopicity was lower than that of methyl methacrylate, It is widely used in the manufacture of high performance acrylic resin and acrylic emulsion, the preparation of light curing adhesives and water-based adhesives.
Synthetic routes
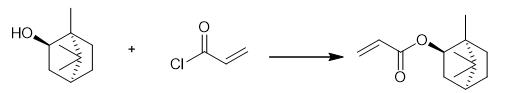
Fig. 2 The synthetic method 1 of Isobornyl acrylate.
Dissolve (-)- borneol (1.00 g, 6.48 mmol, 1 equiv) and triethylamine (0.98 g, 9.72 mmol, 1.5 equiv) in anhydrous THF (20 mL) in a 100 mL round-bottom flask. Add acryloyl chloride (0.88 g, 9.72 mmol, 1.5 equiv) dropwise to the reaction mixture at 0 °C. Allow the reaction to stir at room temperature overnight. Filter the reaction solution. Concentrate the reaction solution on the rotary evaporator. Wash the mixture with water (20 mL). Extract the mixture with dichloromethane (3x20 mL). Dry the combined organic phases over anhydrous sodium sulfate. Filter and concentrate the residue. Purify the crude product further by silica gel column chromatography (petroleum ether/ethyl acetate = 4:1) to obtain Isobornyl acrylate[1].
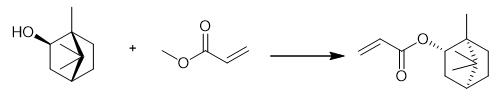
Fig. 3 The synthetic method 2 of Isobornyl acrylate.
Add methyl acrylate (1.26 mL, 14 mmol) to a mixture of magnesium 2, 6-di-tert-butyl-4-methylphenolate (0.10 mmol), activated 5â« molecular sieves (powder), copper (II) dimethyldithiocarbamate (0.0040 mmol) as a polymerization inhibitor and dimethyl sulfone (0.20 mmol) at 25°C for 3 min. Add alcohol (2.0 mmol) to the mixture at 25°C. Stir the mixture at 25°C for 24 h. Purify the resultant crude mixture by silica gel column chromatography (eluent: n-hexane and EtOAc). Chromatograph (eluent: n-hexane and EtOAc). 1H NMR (400 MHz, CDCl3) δ 0.85 (d, J = 3.2 Hz, 6H), 1.01 (s, 3H), 1.07-1.21 (m, 2H), 1.57 (m, 1H), 1.67-1.87 (m, 4H), 4.75 (dd, J = 7.3, 3.7 Hz, 1H), 5.79 (dd, J = 10.5, 1.4 Hz, 1H), 6.09 (dd, J = 17.4, 10.5 Hz, 1H), 6.34 (dd, J = 17.4, 1.4 Hz, 1H). 13C NMR (100 MHz, CDCl3) δ 11.5, 19.9, 20.2, 27.1, 33.8, 38.8, 45.1, 47.0, 48.9, 81.2, 129.3, 130.0, 165.8. IR (neat) 2956, 2879, 1720, 1636, 1619, 1455, 1406, 1390, 1296, 1276, 1199, 1109 cm-1. HRMS (ESI+) calcd for C13H20NaO2 [M+Na]+ 231.1356, found 231.1353 [2].
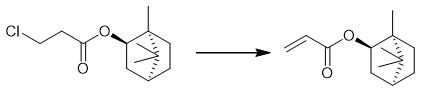
Fig. 4 The synthetic method 3 of Isobornyl acrylate.
Dissolve (1S,2R,4S)-1,7,7-trimethylbicyclo[2.2.1]- heptan-2-yl 3-chloropropanoate (0.30 g, 1.4 mmol) in acetone. Add Et3N (0.3 ml, 2 mmol) to the reaction mixture. Stir the reaction mixture at 40°C for 18 hours. Remove the acetone under reduced pressure. Add brine to the reaction mixture. Extract the reaction mixture with ethyl acetate (3x10 ml). Dry the extract over anhydrous Na2SO4. Remove the solvent under reduced pressure to obtain (1S,2R,4S)-1,7,7-trimethylbicyclo[2.2.1]heptan-2-yl acrylate. 1H NMR δ, ppm (J, Hz): 0.82 (3H, s, 9-CH3); 0.85 (3H, s, 10-CH3); 0.89 (3H, s, 8-CH3); 0.99 (1H, dd, 2J = 13.7, J2endo,1exo= 3.5, 2-CHendo); 1.18-1.34 (2H, m, 4-CHendo, 5-CHexo); 1.63-1.78 (2H, m, 3-CH, 4-CHexo); 1.9-2.0 (1H, m, 5-CHendo); 2.30-2.42 (1H, m, 2-CHexo); 4.90-4.95 (1H, m, 1-CHexo); 5.78 (1H, dd, 2J = 1.6, 3J = 10.5, CH=CHcis); 6.12 (1H, dd, 3Jtrans= 17.3, 3Jcis= 10.5, CH=CH2); 6.36 (1H, dd, 2J = 1.6, 3J = 17.3, CH=CHtrans). 13C NMR δ, ppm: 166.4 (s); 129.9 (t); 128.9 (d); 79.9 (d); 48.7 (s); 47.7 (s); 44.8 (d); 36.6 (t); 28.2 (t); 27.9 (t); 27.0 (t); 19.6 (q); 18.7 (q); 13.4 (q) [3].
Hazard
Allergic contact dermatitis
Background: Acrylates constitute an important cause of occupational contact dermatitis. Isobornyl acrylate sensitization has been reported in only 2 cases.
Background. Glucose sensors, such as FreeStyle (R) Libre, are innovative medical devices developed for diabetes patients as a replacement for classic glucose meters, ensuring continuous glucose monitoring without the disadvantage of regular skin finger pricks. Objectives. To report several cases of allergic contact dermatitis caused by FreeStyle (R) Libre, and to report on isobornyl acrylate as a culprit allergen. Patients and Methods. Fifteen patients presented with allergic contact dermatitis caused by FreeStyle (R) Libre. All but 1 were patch tested with a baseline series, and with pieces and/or ultrasonic bath extracts of (the adhesive part of) the glucose sensor. Isobornyl acrylate was patch tested, in various concentrations and vehicles, in 13 patients. Gas chromatography-mass spectrometry (GC-MS) of the sensors was performed. Results. All patients reacted to the adhesive part of the sensor, and 12 patients were shown to be sensitized to isobornyl acrylate. Simultaneous reactions to other allergens were rarely observed. GC-MS showed the presence of isobornyl acrylate in the sensors. Conclusions. Cases of allergic contact dermatitis caused by FreeStyle (R) Libre are increasingly being observed, and isobornyl acrylate is a relevant culprit allergen. Cross-reactivity to other acrylates was infrequently observed, but other, hitherto unidentified, contact allergens may still be present in the device [5].
References
[1] Cheng Q, Guo X, Hao X, et al. Fabrication of robust antibacterial coatings based on an organic–inorganic hybrid system[J]. ACS applied materials & interfaces, 2019, 11(45): 42607-42615.
[2] Ng J Q, Arima H, Mochizuki T, et al. Chemoselective Transesterification of Methyl (Meth) acrylates Catalyzed by Sodium (I) or Magnesium (II) Aryloxides[J]. ACS catalysis, 2020, 11(1): 199-207.
[3] Sokolova A S, Yarovaya O I, Shtro A A, et al. Synthesis and biological activity of heterocyclic borneol derivatives[J]. Chemistry of Heterocyclic Compounds, 2017, 53(3): 371-377.
[4] Christoffers W A, Coenraads P J, Schuttelaar M L A. Two decades of occupational (meth) acrylate patch test results and focus on isobornyl acrylate[J]. Contact Dermatitis, 2013, 69(2): 86-92.
[5] Herman A, Aerts O, Baeck M, et al. Allergic contact dermatitis caused by isobornyl acrylate in Freestyle (R) Libre, a newly introduced glucose sensor[J]. Contact Dermatitis, 2017, 77(6): 367-373.
You may like
Related articles And Qustion
See also
Lastest Price from Isobornyl acrylate manufacturers

US $16.00-14.00/kg2025-07-18
- CAS:
- 5888-33-5
- Min. Order:
- 1000kg
- Purity:
- ≥99.2%
- Supply Ability:
- 120 tons

US $0.00-0.00/kg2025-06-13
- CAS:
- 5888-33-5
- Min. Order:
- 1kg
- Purity:
- 99.0% min
- Supply Ability:
- 10tons