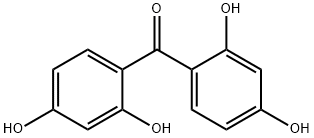
Synthesis and Applications of 2,2',4,4'-Tetrahydroxybenzophenone
General description
2,2',4,4'-Tetrahydroxybenzophenone, a distinctive derivative of benzophenone, stands out due to its specialized structural formula, comprising four hydroxyl groups strategically positioned on the benzophenone skeleton. This molecular configuration endows the compound with exceptional chemical properties, such as a heightened ability to absorb and dissipate ultraviolet (UV) light, making it highly versatile and invaluable across a multitude of applications. Its utility spans from polymer chemistry, where it serves as an integral UV stabilizer, safeguarding polymers from UV-induced degradation and extending their functional lifespan, to the cosmetics industry, where its efficacy in absorbing harmful UV rays translates into robust sun protection formulations. Furthermore, its role in UV light stabilization is critical, not just in protecting materials and the skin from the deleterious effects of UV radiation, but also in enhancing the performance and durability of products exposed to sunlight. This unique blend of properties positions 2,2',4,4'-Tetrahydroxybenzophenone as a crucial ingredient in developing advanced materials and formulations designed for optimal protection against UV damage.
Synthesis
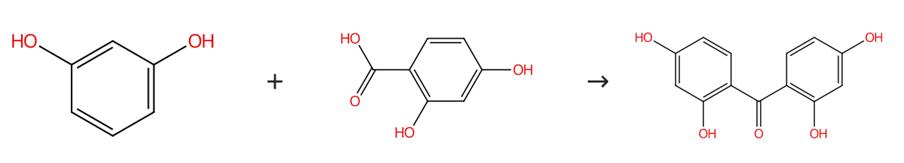
Fig. 1 The synthesis route of 2,2',4,4'-Tetrahydroxybenzophenone
Add anhydrous zinc chloride (27.2 g) and phosphorus oxychloride (25 mL) to the mixture of resorcinol (50 mmol) and polyhydroxy benzoic acid (55.6 mmol). Reflux the reaction mixture for 2.5 hours in the water bath of 70 °C with stirring. Add the mixture to ice. Cool the mixture to 4 °C for over 24 hours. Filter the precipitate solid. Wash the precipitate solid with 3% sodium bicarbonate twice. Purify the product by recrystallization from boiling water. The synthesis route is shown in Fig. 1[1].
Applications
In the field of polymer chemistry, 2,2',4,4'-Tetrahydroxybenzophenone serves as an effective UV stabilizer and absorber. Polymers, when exposed to UV light, can undergo degradation, leading to a loss of mechanical properties, discoloration, and decreased lifespan of the material. The incorporation of 2,2',4,4'-Tetrahydroxybenzophenone into polymer matrices helps absorb harmful UV radiation, converting it into less harmful thermal energy, thus preventing the degradation of polymers. This property is particularly beneficial in extending the durability and efficacy of outdoor materials and products, such as automotive parts, outdoor furniture, and packaging materials, against UV-induced aging.
In cosmetics and skincare products, 2,2',4,4'-Tetrahydroxybenzophenone is valued for its ability to protect the skin from UV damage. As an ingredient in sunscreens and other personal care products, it functions by absorbing UV radiation, preventing it from penetrating the skin where it can cause sunburn, premature aging, and increase the risk of skin cancer. Its effectiveness in filtering out UV rays makes it a crucial component in formulations designed to shield the skin from the harmful effects of sun exposure, thereby contributing to skin health and cosmetic appeal[2].
Moreover, 2,2',4,4'-Tetrahydroxybenzophenone finds application in the production of photochromic materials. These materials change color in response to UV light exposure, a property that is useful in the development of sunglasses, windows, and lenses that automatically adjust to varying light conditions. The ability of 2,2',4,4'-Tetrahydroxybenzophenone to undergo reversible structural changes upon exposure to UV light is exploited to create materials that protect against glare and high-intensity sunlight, enhancing visual comfort and safety.
Another interesting application of 2,2',4,4'-Tetrahydroxybenzophenone is in the field of organic electronics, particularly in organic photovoltaic (OPV) cells and organic light-emitting diodes (OLEDs). Its strong UV absorption capabilities can be utilized to protect these devices from UV-induced degradation, thereby improving their stability and operational lifespan. This is particularly important for the longevity and efficiency of organic electronic devices, which are susceptible to damage from UV radiation.
In conclusion, 2,2',4,4'-Tetrahydroxybenzophenone is a multifaceted compound with significant applications across various industries. Its ability to absorb and stabilize against UV radiation makes it invaluable in polymer chemistry for protecting materials from UV degradation. In the cosmetic industry, it plays a critical role in safeguarding the skin from UV damage, while its use in photochromic materials offers innovative solutions for light-sensitive applications. Additionally, its potential in organic electronics highlights its importance in the advancement of UV protection technologies. The diverse applications of 2,2',4,4'-Tetrahydroxybenzophenone underscore its utility in enhancing the durability, safety, and functionality of a wide range of products.
References
[1] Wu, Jianlong; et al.Synthesis and biological evaluation of polyhydroxy benzophenone as mushroom tyrosinase inhibitors.Journal of Enzyme Inhibition and Medicinal Chemistry (2011), 26(3), 449-452.
[2]Dorman G, Prestwich G D. Benzophenone photophores in biochemistry[J]. Biochemistry, 1994, 33(19): 5661-5673.
General description
2,2',4,4'-Tetrahydroxybenzophenone, a distinctive derivative of benzophenone, stands out due to its specialized structural formula, comprising four hydroxyl groups strategically positioned on the benzophenone skeleton. This molecular configuration endows the compound with exceptional chemical properties, such as a heightened ability to absorb and dissipate ultraviolet (UV) light, making it highly versatile and invaluable across a multitude of applications. Its utility spans from polymer chemistry, where it serves as an integral UV stabilizer, safeguarding polymers from UV-induced degradation and extending their functional lifespan, to the cosmetics industry, where its efficacy in absorbing harmful UV rays translates into robust sun protection formulations. Furthermore, its role in UV light stabilization is critical, not just in protecting materials and the skin from the deleterious effects of UV radiation, but also in enhancing the performance and durability of products exposed to sunlight. This unique blend of properties positions 2,2',4,4'-Tetrahydroxybenzophenone as a crucial ingredient in developing advanced materials and formulations designed for optimal protection against UV damage.
Synthesis

Fig. 1 The synthesis route of 2,2',4,4'-Tetrahydroxybenzophenone
Add anhydrous zinc chloride (27.2 g) and phosphorus oxychloride (25 mL) to the mixture of resorcinol (50 mmol) and polyhydroxy benzoic acid (55.6 mmol). Reflux the reaction mixture for 2.5 hours in the water bath of 70 °C with stirring. Add the mixture to ice. Cool the mixture to 4 °C for over 24 hours. Filter the precipitate solid. Wash the precipitate solid with 3% sodium bicarbonate twice. Purify the product by recrystallization from boiling water. The synthesis route is shown in Fig. 1[1].
Applications
In the field of polymer chemistry, 2,2',4,4'-Tetrahydroxybenzophenone serves as an effective UV stabilizer and absorber. Polymers, when exposed to UV light, can undergo degradation, leading to a loss of mechanical properties, discoloration, and decreased lifespan of the material. The incorporation of 2,2',4,4'-Tetrahydroxybenzophenone into polymer matrices helps absorb harmful UV radiation, converting it into less harmful thermal energy, thus preventing the degradation of polymers. This property is particularly beneficial in extending the durability and efficacy of outdoor materials and products, such as automotive parts, outdoor furniture, and packaging materials, against UV-induced aging.
In cosmetics and skincare products, 2,2',4,4'-Tetrahydroxybenzophenone is valued for its ability to protect the skin from UV damage. As an ingredient in sunscreens and other personal care products, it functions by absorbing UV radiation, preventing it from penetrating the skin where it can cause sunburn, premature aging, and increase the risk of skin cancer. Its effectiveness in filtering out UV rays makes it a crucial component in formulations designed to shield the skin from the harmful effects of sun exposure, thereby contributing to skin health and cosmetic appeal[2].
Moreover, 2,2',4,4'-Tetrahydroxybenzophenone finds application in the production of photochromic materials. These materials change color in response to UV light exposure, a property that is useful in the development of sunglasses, windows, and lenses that automatically adjust to varying light conditions. The ability of 2,2',4,4'-Tetrahydroxybenzophenone to undergo reversible structural changes upon exposure to UV light is exploited to create materials that protect against glare and high-intensity sunlight, enhancing visual comfort and safety.
Another interesting application of 2,2',4,4'-Tetrahydroxybenzophenone is in the field of organic electronics, particularly in organic photovoltaic (OPV) cells and organic light-emitting diodes (OLEDs). Its strong UV absorption capabilities can be utilized to protect these devices from UV-induced degradation, thereby improving their stability and operational lifespan. This is particularly important for the longevity and efficiency of organic electronic devices, which are susceptible to damage from UV radiation.
In conclusion, 2,2',4,4'-Tetrahydroxybenzophenone is a multifaceted compound with significant applications across various industries. Its ability to absorb and stabilize against UV radiation makes it invaluable in polymer chemistry for protecting materials from UV degradation. In the cosmetic industry, it plays a critical role in safeguarding the skin from UV damage, while its use in photochromic materials offers innovative solutions for light-sensitive applications. Additionally, its potential in organic electronics highlights its importance in the advancement of UV protection technologies. The diverse applications of 2,2',4,4'-Tetrahydroxybenzophenone underscore its utility in enhancing the durability, safety, and functionality of a wide range of products.
References
[1] Wu, Jianlong; et al.Synthesis and biological evaluation of polyhydroxy benzophenone as mushroom tyrosinase inhibitors.Journal of Enzyme Inhibition and Medicinal Chemistry (2011), 26(3), 449-452.
[2]Dorman G, Prestwich G D. Benzophenone photophores in biochemistry[J]. Biochemistry, 1994, 33(19): 5661-5673.
Related articles And Qustion
Lastest Price from 2,2',4,4'-Tetrahydroxybenzophenone manufacturers
US $0.00-0.00/KG2025-07-05
- CAS:
- 131-55-5
- Min. Order:
- 1KG
- Purity:
- 99.0%
- Supply Ability:
- 10000KGS

US $0.00-0.00/G2025-06-11
- CAS:
- 131-55-5
- Min. Order:
- 0.1G
- Purity:
- 99.99%
- Supply Ability:
- 2000000T