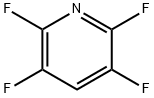
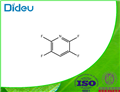
Synthesis and Application of Tetrafluoropyridine
Pyridine and its derivatives have been developed and utilized relatively early at home and abroad. It is widely used in medicine, pesticides, rubber, dyes and other fields. Since the 1960s, there have been hundreds of medicines, pesticides, dyes, and some special reagents developed with fluorine-containing aromatic compounds as intermediates[1]. In the weakly basic pyridine ring, the nitrogen atom is more negatively charged, and then a fluorine atom with a strong electronegativity is introduced to synthesize a fluorine-containing pyridine intermediate[2]. Some compounds developed by it have special functions and biological activities Has attracted the attention of chemists. In the past ten years, a variety of fluorine-containing pyridine compounds have been synthesized successively, and have been applied in the fields of pesticides and medicine.
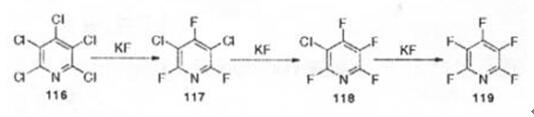
Under normal circumstances, tetrafluoro and polyfluoropyridines are generally obtained by reducing or nucleophilic halogen exchange instead of perhalopyridines. However, at a high temperature of 220 °C, potassium fluoride is used as a fluorinating agent, sulfolane is used as a solvent, 2,3,5-trichloropyridine exchanged by fluorine halide, only partially fluorinated to generate 2,3-difluoro-5-chloropyridine[3]. Pentachloropyridine is used as the raw material of polyfluoropyridine. At higher temperature, the corresponding tetrafluoropyridine is finally formed through multiple steps of fluorination[4].
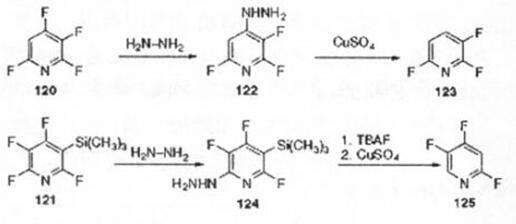
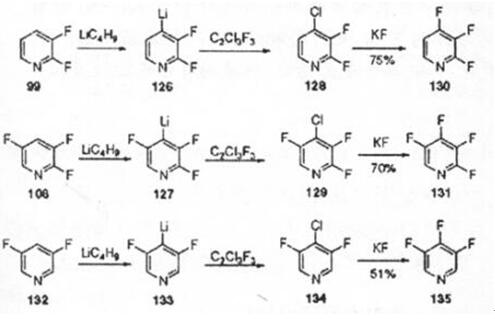
Studies have provided a new method for the synthesis of tetrafluoropyridine. This method uses difluoro or trifluoropyridine as a raw material, first deprotonated by n-butyllithium to generate the corresponding lithium pyridine reagent, and then converted to chlorofluoropyridine. Finally, in an aprotic solvent such as dimethyl sulfoxide, spray-dried potassium fluoride is used as a fluorinating agent, and the fluorine-halogen exchange is performed at a certain temperature to generate the corresponding tetrafluoropyridine[5]. Polyfluoropyridine is a commonly used fluorinated building block in the synthesis of organic fluorine heterocycles. It is often used in the synthesis of many drugs and has a wide range of applications[6].
Many fluorine-containing pyridine compounds have been widely used, especially in the research and development of drugs and new drugs. Therefore, it is still meaningful to study some new methods for synthesizing fluorine-containing pyridine compounds[7]. Compounds are still a serious challenge and an important subject of organic fluorine chemistry.
References
[1] Christopher A. Hargreaves, Graham Sandford, Rachel Slater. Synthesis of Tetrahydropyrido[2,3-b]pyrazine Scaffolds from 2,3,5,6-Tetrafluoropyridine Derivatives[J]. Cheminform, 2007, 63(24):5204-5211.
[2] Aurelie Baron, Graham Sandford, Rachel Slater. Polyfunctional Tetrahydropyrido[2,3‐b]pyrazine Scaffolds from 4‐Phenylsulfonyl Tetrafluoropyridine[J]. Journal of Organic Chemistry, 2006, 37(12):no-no.
[3] Paul L Coe, Anthony J Rees. ChemInform Abstract: Preparation and Reactions of 2,3,4,6-Tetrafluoropyridine and Its Derivatives[J]. Journal of Fluorine Chemistry, 2000, 101(1):45-60.
[4] Richard D Chambers, Ali Khalil, Paul Richmond. Polyhalogenoheterocyclic compounds: Part 51. [1] Macrocycles from 4-alkoxy-tetrafluoropyridine derivatives[J]. 125(5):715-720.
[5] Reza Ranjbar-Karimi, Mirrasoul Mousavi. Regiochemistry of nucleophilic substitution of 4-phenylsulfonyl tetrafluoropyridine with unequal bidentate nucleophiles[J]. 131(5):587-591.
See also
Lastest Price from 2,3,5,6-Tetrafluoropyridine manufacturers
US $1.10/g2025-04-17
- CAS:
- 2875-18-5
- Min. Order:
- 1g
- Purity:
- 99.0% min
- Supply Ability:
- 100 tons min

US $0.00-0.00/KG2025-04-04
- CAS:
- 2875-18-5
- Min. Order:
- 1KG
- Purity:
- 98%
- Supply Ability:
- 1ton