Synthesis and Application of Propionyl chloride
General description
Propionyl chloride is a colorless liquid with a pungent odor. It is soluble in ethanol. It is used as a propionylation reagent in organic synthesis and is an intermediate for the preparation of various propionic acid derivatives, such as propiophenone, etc.; it is used in the production of pesticide propanil; in the pharmaceutical industry, it is used in the production of antiepileptic drugs dimethoin, Cholesterol, anti-adrenergic drug methoxyamine hydrochloride, etc. Propionyl chloride is an intermediate for the plant growth regulator prohexadione.
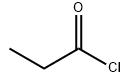
Fig. 1 The structure of Propionyl chloride.
Preparation
Propionyl chloride can be prepared by reaction of propionic acid with phosphorus trichloride, phosphorus pentachloride, sulfoxide chloride or phosgene.
1. Put propionic acid and phosphorus trichloride into the reaction pot, add a number of magnetic chips, and reflux reaction at 50℃ for 6h. Stand for 1-2H, divide the lower phosphite, that is, propionyl chloride. Under the condition of 1.32:1 propionic acid: PCl3, the yield was 95%. When the crude product needs to be purified, the distillation method can be used to collect the fraction at about 80℃ to obtain the finished product. Raw material consumption quota: propionic acid (99.5%) 990kg/t, phosphorus trichloride (98%) 690kg/t.
2. Benzoyl chloride method by propionic acid and benzoyl chloride action.
Application
Influence of reaction kinetics in acylation reaction
The kinetics of 1-(2-phenylethyl)-4-phenylaminopiperidine acylation with propionyl chloride in nonaqueous solutions to form fentanyl hydrochloride have been studied experimentally using high-performance liquid chromatography (HPLC). It is established that the reaction is second order. The kinetics and activation parameters of the reaction have been determined [1].
Photodissociation
Photofragment translational spectroscopy was used to study the photodissociation of propionyl chloride at 248 nm. The crossed laser-molecular beam experiment with VUV photoionization showed two primary dissociation channels, C-Cl bond fission and HCl elimination. Following cleavage of the C-Cl bond, unimolecular dissociation of the propionyl radical produced CH3CH2 and CO. The energy barrier to the CH3CH2CO --> CH3CH2 + CO reaction was estimated to be in the range of 16.3 +/- 1.5 kcal/mol by determining the internal energy distribution of surviving propionyl radicals. No other secondary dissociation channels were observed for the propionyl radical. The HCl elimination channel, previously reported only for the condensed phase of propionyl chloride, was observed as the minor primary dissociation channel in the gas phase. The cofragment to the HCl elimination, CH3CHCO or CH2CH2CO, underwent secondary dissociation to produce CO and CH2CH2 with a significant amount of energy partitioned into translational motion [2].
UV photodissociation
Winter et al. have investigated the photodecomposition of propionyl chloride, CH3CH2COCl, in an argon matrix at 10 K using FTIR absorption spectroscopy. The decomposition products formed following irradiation at 266, 254, or 248 nm are methyl ketene, CH3CH=CO, and HCl; no other photoproducts are observed. We have carried out FTIR polarization studies to determine the relative orientation of the photoproducts and found that the HCl molecule is situated perpendicular to the carbonyl group in the methyl ketene. The orientation in the photoproducts and kinetic studies of the acid chloride dissociation point to a direct elimination mechanism for propionyl chloride decomposition. This is consistent with the direct mechanism proposed for acetyl chloride, CH3COCl, photodissociation in both an argon matrix and the solid crystalline form. We have calculated detailed thermodynamics for CH3COCl and CH3CH2COCl decomposition and found them consistent with the proposed elimination mechanism. Winter et al. also assign the fundamental vibrational frequencies for the methyl ketene-HCl complex on the basis of ab initio calculations and polarization studies [3].
Precautions for the experiment
1. Before the experiment, wear protective glasses, protective clothing, mask, and gloves, and avoid contact with skin.
2. If toxic or irritating substances and harmful substances are encountered during the experiment, the experimental operation should be completed in the glove box when necessary to avoid causing harm to the experimenter.
3. The pipetting nozzle for taking samples should be replaced in time. If necessary, the filter cartridge suction head should be selected as far as possible to avoid cross contamination.
4. When weighing drugs, use weighing paper, take drugs and weigh them in a place without wind to avoid spreading. The container of reagents must be clean and disinfected before use.
5. When taking medicine, try to use multiple medicine spoons separately, clean them after use, dry them, disinfect them and store them.
6. Waste generated after the experiment shall be classified and stored and handed over to a professional biological waste gas treatment company to avoid environmental pollution.
References
[1] Murashova U A, Skalkina L V, Glukhan E N, et al. Kinetics of 1-(2-phenylethyl)-4-phenylaminopiperidine acylation by propionyl chloride in nonaqueous media[J]. Pharmaceutical chemistry journal, 2010, 44(5): 261-264.
[2] McCunn L R, Krisch M J, Takematsu K, et al. Competing Pathways in the 248 nm Photodissociation of Propionyl Chloride and the Barrier to Dissociation of the Propionyl Radical[J]. The Journal of Physical Chemistry A, 2004, 108(39): 7889-7894.
[3] Winter P R, Rowland B, Hess W P, et al. UV photodissociation of matrix-isolated propionyl chloride[J]. The Journal of Physical Chemistry A, 1998, 102(19): 3238-3248.
See also
Lastest Price from Propionyl chloride manufacturers

US $0.00-0.00/kg2025-04-21
- CAS:
- 79-03-8
- Min. Order:
- 1kg
- Purity:
- 99.99%
- Supply Ability:
- 20 tons

US $2.00/kg2024-12-17
- CAS:
- 79-03-8
- Min. Order:
- 10000kg
- Purity:
- 99%
- Supply Ability:
- 10000000
![943516-54-9 6,6-Dimethyl-3-azabicyclo[3.1.0]hexane; Synthesis; Application](/NewsImg/2022-08-29/6379736644807646651947709.jpg)