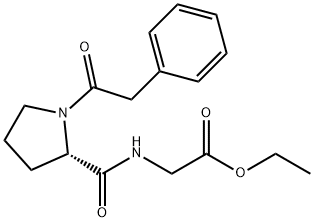
Synthesis and Application of Noopept
Generally speaking
The trade name of N-(1-(phenylacetyl)-L-prolyl)glycine ethyl ester is NOOPEPT as a treatment for diseases such as obesity, alcohol-dependent degeneration or toxic injury.
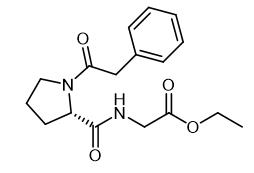
Fig. 1 The structure of Noopept.
Synthetic routes
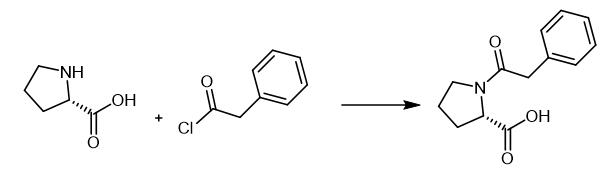
Fig. 2 The synthetic step 1 of Noopept.
Add phenylacetyl chloride (4.03 g, 26 mmol) to a mixture of reactant (3.0 g, 26 mmol) and dichloromethane (40 mL) using syringe. Stir continuously for 2 h at 0~5 °C in an ice bath. Add K2CO3 (2.16 g, 15.6 mmol) and hexadecyl trimethyl ammonium bromide (0.15 g, 5 mol%) into the reaction solution in batches and reflux for 4.5 h. Monitor the reaction by TLC. Cool the solution to room temperature to separate the precipitation out. Wash the filtrate twice with ice water (2 × 10 mL) and remove the solvent by a rotary evaporator. Acidify the aqueous solution to pH = 1~3 with 10 mL 1% HCl and purify by recrystallization with ethyl acetate. 1H NMR spectrum in CDCl3: δ = 2.10 (d d, 4H CβH2-CγH2 proline, J = 7.9 Hz); 3.48~3.59 (d q, 2H CδH2 proline, J = 4.4 Hz); 3.75 (s, 2H CH2-C6H5); 4.63 (d, 1H CαH proline); 7.25 ~ 7.37 (m, 5H CH2-C6H5); 11.38 (br s, 1H COOH). 13C NMR spectrum in CDCl3: δ = 173.50, 173.29, 173.97, 129.67, 129.45, 129.32, 127.94, 127.74, 127.72, 77.84, 77.63, 60.77, 60.55, 48.73, 48.53, 42.50, 42.28, 28.32, 27.97, 25.40 [1].
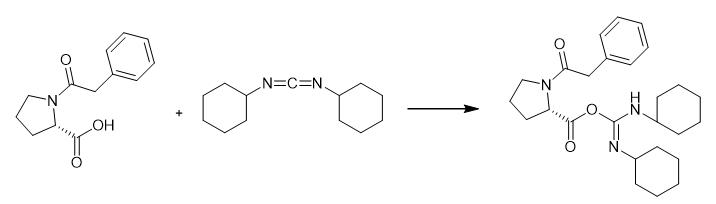
Fig. 3 The synthetic step 2 of Noopept.
Perform the dehydration and condensation of N-phenylacetyl-L-proline and ethyl glycine under the action of DCC/DMAP. Add DCC (1.77 g, 8.6 mmol) and DMAP (0.03 g, 3 mol%) to N-phenylacetyl-L-proline (2 g, 8.6 mmol) dissolved in a 50 mL mixed solvent of THF and dichloromethane (1:1; THF/dichloromethane (v/v)). Stir the mixture overnight at 0~5 °C [1].
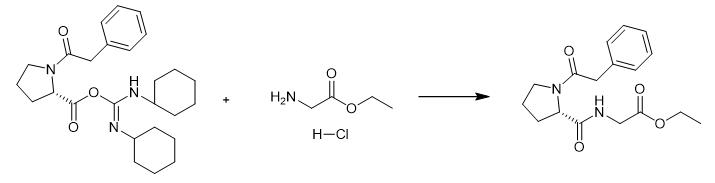
Fig. 3 The synthetic step 3 of Noopept.
Add a mixture of glycine ethyl ester hydrochloride (1.2 g, 8.6 mmol), Et3N (0.87 g, 8.6 mmol) and hexadecyl trimethyl ammonium bromide (0.16 g, 5 mol%) dissolved in dichloromethane (20 mL) and stir for 48 h at room temperature. Filter the precipitate and evaporate the solvent in vacuum. Purify the residue by recrystallization from ethanol to obtain product. 1H NMR spectrum in DMSO-d6: δ = 1.18 (t, 5H CH3CH2). 1.83~1.99 (m, 4H CβH2-CγH2 proline, J = 7.9 Hz); 3.35 (m, 2H CαH2 proline,J = 4.4 Hz); 3.67 (s, 2H CH2-C6H5). 3.80 (d, 2H CαH2 Gly, J = 5.9 Hz); 4.08 (q, 2H CH3-CH2-O, 55%); 4.09 (q, 2H CH3-CH2-O, 45%); 4.32 (dd, 1H CαH proline, 55%); 4.47 (dd, 1H CαH proline, 45%); 7.18~7.24 (m, 5H CH2-C6H5); 8.28 (t, 1H NH Gly, J = 5.9 Hz 55%); 8.63 (t, 1H NH Gly, J = 5.9 Hz 45%). 13C NMR spectrum in DMSO-d6: δ = 173.1, 172.7, 170.3, 170.2, 169.8, 169.5, 136.0, 130.0, 129.9, 128.6, 128.5, 126.8, 126.7, 61.0, 60.9, 60.3, 59.8, 47.6, 47.0, 41.3, 41.2, 41.1, 40.9, 32.1, 29.9, 24.5, 22.7, 14.5 [1].
Pharmacological properties
Ostrovskaya et al. describe pharmacological properties of the new nootropic drug noopept created using an original approach based on the imitation of a nonpeptide nootrope structure by means of the short- peptide design. In particular, the structure of pyracetam was designed using dipeptide nootropes. Experimental investigations of noopept (N-phenylacetyl-L-polyglycine ethyl ester) showed that the new drug exceeds pyracetam both with respect to the effective dose level (1000 times lower for noopept than for pyracetam) and in the spectrum of mnemotropic activity. In contrast to pyracetam facilitating only the early stages of the memory process, noopept positively influences the memory consolidation and retrieval steps as well. The new drug produces an additional selective anxiolytic action. The pronounced neuroprotective effect of noopept was demonstrated both in vivo (in cases of various forms of brain ischemia) and in vitro (on various neuronal models). The drug action is based on the antioxidant effect, the antiinflammatory action, and the ability to inhibit the neurotoxicity of excess calcium and glutamate, and to improve the blood rheology. It was established for the first time that the activity of noopept is retained both upon parenteral introduction and upon peroral administration, which is a principal advantage of this proline-containing dipeptide over other, more complex peptides. This property provided a basis for the development of a medicinal form of noopept for peroral usage. At present, noopept tablets (noopept 5 and 10 mg) are under clinical assessment as a means of treating cognitive deficiency of cerebrovascular and post-traumatic origin [2].
Anti-diabetic
Type 1 diabetes (T1DM) is a common chronic disease in childhood. Increasing insulin resistance in puberty gives rise to higher doses of insulin usage in treatment. Of this reason new approaches in treatment are needed. Noopept researches suggest it to have anti-diabetic properties. We tried to determine the effects of noopept on pubertal diabetes. The research was made with 60 prepubertal, 28 day-old, male, Sprague Dawley rats. The rats were divided into randomised 6 groups (n = 10/group). i) Control, ii) Diabetes Control, iii) Noopept Control, iv) Diabetes + Noopept, v) Diabetes + Insulin, vi) Diabetes + Insulin + Noopept. T1DM model was induced by streptozotocin on postnatal 28th day. 0.5 mg/kg noopept and 1 IU insulin were administered intraperitoneally for 14 days. Blood glucose and body weight measurements, puberty follow-up and MWM tests were performed. Hippocampus, hypothalamus and testis were evaluated histologically. Hypothalamic GnRH and kisspeptin were studied immunohistochemically. Serum LH, FSH and insulin, hippocampal homogenate NGF and BDNF levels were determined by ELISA. Delayed puberty was normalized by noopept (p < 0.05). Blood glucose levels were lower in noopept-administered diabetic groups (p < 0.05). Noopept decreased HOMA-IR in insulin administered diabetic group (p < 0.05). Number of degenerated cells in hippocampus and testis were higher in diabetes control group when compared with other groups (p < 0.05). GnRH immunoreactivity in Diabetes + Noopept group was increased when compared to insulin + noopept group (p = 0.018). There was no difference in kisspeptin, serum LH, FSH, hippocampal NGF-BDNF levels and spatial learning assessment among groups (p > 0.05) [3].
Cognitive Enhancer
The effect of noopept (N-phenylacetyl-L-prolyl-glycine ethyl ester) on the DNA-binding activity of HIF-1 in SH-SH5Y cells and the mechanisms of stabilization of this transcription factor were studied in vitro. Noopept was shown to increase both the basal DNA-binding activity of HIF-1 and the activity induced by various hypoxia mimetics. The mechanism of stabilization of the oxygen-sensitive HIF1 alpha subunit by
Related articles And Qustion
See also
Lastest Price from Noopept manufacturers

US $0.60-0.34/g2025-10-31
- CAS:
- 157115-85-0
- Min. Order:
- 1g
- Purity:
- 99%
- Supply Ability:
- 300tons

US $10.00/ASSAYS2025-05-04
- CAS:
- 157115-85-0
- Min. Order:
- 1ASSAYS
- Purity:
- 99%
- Supply Ability:
- 10 ton