Synthesis and Application of 3,5-dichlorobenzoic acid
Generally speaking
3,5-Dichlorobenzoic acid is a brown powder with a melting point of 184-187 °C. The results of acute toxicity test showed that the subcutaneous LD50 of mice was 250 mg/kg; the abdominal cavity LD50 of mice was 237 mg/kg.
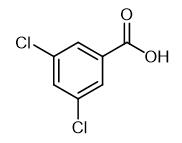
Fig. 1 The structure of 3,5-Dichlorobenzoic acid.
Synthetic routes
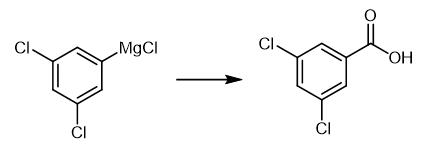
Fig. 2 The synthetic method 1 of 3,5-Dichlorobenzoic acid.
The freshly prepared and titrated (3,5-dichlorophenyl)-magnesium chloride (1.54 mL, 1 mmol) prepared according to TP1 (30 min, 0.65 M, 73%), was added to a flask filled with anhyd CO2 (g). Then, anhyd CO2 (g)was bubbled through the reaction mixture (ca. 5 min) until a balloon attached to the reaction flask by a short length rubber tubing and a needle adapter was inflated. The reaction mixture was stirred for 1 h at 25°C and then diluted with Et2O (15 mL) and extracted with sat. aq NaHCO3 (3 x 20 mL). The combined aq phases were carefully acidified with HCl (5 mL) until pH <5 and extracted with Et2O (3x30 mL). The combined organic layers were dried over MgSO4 and concentrated in vacuo. The compound as a yellow solid (189 mg, 99%) [1].
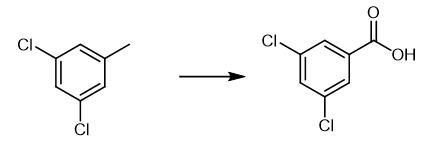
Fig. 3 The synthetic method 2 of 3,5-Dichlorobenzoic acid.
Charge 25mL quartaz glass tube with a stiring bar. Add substituted toulene (0.1 mmol), CeCl3 (5 mol%, 0.0012 g), CCl3CH2OH 1.0 equiv, 0.0149 g), CH3CN (2 mL) to the tube under O2 atmosphere. Place the mixture perpendicular to a 400 nm LED lights (10W). Stir the reaction mixture blue light irradiation for 48 hours at 60°C. Indicate the completion of reaction by TLC. Add ethyl acetate and water to the mixture to extract for three times. Concentrate the organic phase under reduced pressure to afford 3,5-dichlorobenzoic acid. 1H NMR (400 MHz, DMSO) δ 12.82 (s, 1H), 7.91 (s, 1H), 7.86 (s, 1H), 7.85 (s, 1H). 13C NMR (100 MHz, DMSO) δ 165.4, 135.0, 134.8, 132.7, 128.3 [2].
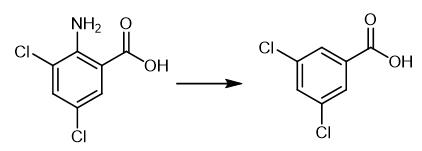
Fig. 3 The synthetic method 3 of 3,5-Dichlorobenzoic acid.
Add solution of amine (5 mmol) in THF (3 mL) dropwise to a solution of r-BuONO (7.5 mmol) and DMSO (0.5 mmol) in THF (7 mL) at 30°C over 20 min. Stir the mixture at 30 °C for 1 h (monitor by TLC). Evaporate the solvent and determine the yields of the low boiling point products by GC. Isolate the high boiling point products by column chromatography on silica gel (hexane/ethyl acetate).1H NMR (400 MHz. CDCl3):δ 7.98 (d. 7 = 2.0 Hz.2H), 7.61 (t. 7 = 2.0 Hz, 1H), 1.27 (s, 1H). MP 187.3-188.5 °C [3].
Application
Synthesis of New Heat-resistant Energetic Material BTAHNAB
Synthesizing heat-resistant energetic material 3,3,5,5-tetraamino-2,2,4,4,6,6-six-nitro-azobenzene into nitric acid mixed acid by using 3, 5-dichlorobenzoic acid as raw material comprises adding 3,5-dichlorobenzoic acid to the mixed acid of nitro-sulfur, slowly raising the temperature, keeping the temperature and stirring at a constant speed, and reacting to obtain 3,5-dichloro-4-nitrobenzoic acid, adding 3,5-dichloro-4-nitrobenzoic acid to sulfuric acid, dropping chloroform, adding sodium azide, slowly raising the temperature, and keeping the temperature at a constant stirring rate to obtain 3,5-dichloro-4-nitroaniline through the reaction, adding sodium nitrate and 3,5-dichloro-4-nitroaniline to sulfuric acid in turn, slowly raising the temperature, keeping the temperature under constant stirring, reacting to obtain 3,3,5,5-tetrachloro-2,2,4,4,6,6-six-nitro-azobenzene, mixing 3,3,5,5-tetrachloro-2,2,4,4,6,6-six-nitro-azobenzene with toluene, and slowly raising the temperature [4].
Crystal structures
The crystal structures of 2,6-dichlorobenzoic acid (1) and 3,5-dichlorobenzoic acid (2) were obtained by single-crystal X-ray diffraction. Crystallization of 1 occurred in the centrosymmetric triclinic space group P1 (No. 2) with a = 7.2678(9), b = 9.8543(8), and c = 11.8290(11) Angstrom; alpha = 95.000(7), = 104.262(10), and gamma = 102.128(8)degrees; and Z = 4. Crystallization of 2 occurred in the monoclinic centrosymmetric space group P2(1)/n (No. 14) with a = 3.7812(3), b = 13.996(2), and c = 14.514(2) Angstrom; = 95.183(8); and Z = 4. Chlorine substituents of four dimeric molecules of 2 formed square channels, ca. 3.54 Angstrom on a side, which run parallel to the crystallographic a axis. Monomers of 1 and 2 formed centrosymmetric dimers via near-linear hydrogen bonds. Details of the structures and spectroscopic results are presented and discussed [5].
References
[1] Dunst C, Knochel P. Selective Mg insertion into substituted mono-and dichloro arenes in the presence of LiCl: a new preparation of boscalid[J]. Synlett, 2011, 22(14): 2064-2068.
[2] Liu F, Shi Z. Visible-Light-Induced Deep Aerobic Oxidation of Alkyl Aromatics[J]. 2021.
[3] Fang L, Qi L, Ye L, et al. Dimethyl sulfoxide-accelerated reductive deamination of aromatic amines with t-BuONO in tetrahydrofuran[J]. Journal of Chemical Research, 2018, 42(11): 579-583.
[4] Jing S, Tan M, Liu Y, et al. Synthesizing heat-resistant energetic material 3,3,5,5-tetraamino-2,2,4,4,6,6-six-nitro-azobenzene into nitric acid mixed acid by using 3, 5-dichlorobenzoic acid as raw material comprises e.g. adding 3,5-dichlorobenzoic acid to mixed acid[P]. Faming Zhuanli Shenqing, 111825567, 2020.
[5] Pinkus A G, Kautz J A, Ahobila-Vajjula P. Crystal structures of 2, 6-and 3, 5-dichlorobenzoic acids: nonbonded Cl??? Cl contacts[J]. Journal of chemical crystallography, 2003, 33(3): 181-186.
See also
Lastest Price from 3,5-Dichlorobenzoic acid manufacturers
US $0.00-0.00/kg2025-11-19
- CAS:
- 51-36-5
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 100tons

US $10.00/ASSAYS2025-08-21
- CAS:
- 51-36-5
- Min. Order:
- 1ASSAYS
- Purity:
- 99%
- Supply Ability:
- 1 ton
![943516-54-9 6,6-Dimethyl-3-azabicyclo[3.1.0]hexane; Synthesis; Application](/NewsImg/2022-08-29/6379736644807646651947709.jpg)