Synthesis and Application of N-Methylcyclohexylamine
Physicochemical properties
N-Methylcyclohexylamine is a colorless transparent liquid with a boiling point of 149 °C and a density of 0.868 g/mL at 25 °C. It is miscible with many organic solvents and slightly soluble in water.
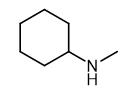
Fig. 1 The structure of N-Methylcyclohexylamine.
Synthetic routes
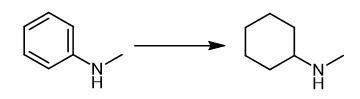
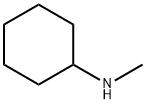
Fig. 2 The synthetic method 1 of N-Methylcyclohexylamine.
Charge a scintillation glass vial containing a small stirrer bar with arenes (1 mmol) and catalyst (0.01 mmol in 40 ?μl stock solution) under nitrogen atmosphere [use 1, 4-dioxane (0.3 mL) as a solvent for solid substrates]. Place the glass vial inside a high-pressure Parr reactor or finger tube pressure reactor, evacuate and pressurize with H2 (30-50 bar) gas. Cool the reactor to room temperature and release the pressurized gas (H2). Analyze the reaction mixture by gas chromatography and1H NMR spectroscopy. Extract the selected crude reaction mixtures with dichloromethane (3 x 10mL). Dry the combined organic phase over sodium sulphate and evaporate the solvent under reduced pressure. Purify the crude product by flash column chromatography. 1H NMR (CDCl3): δ 2.40 (s, 3H, CH3), 2.15 (br. S, 1H, NH), 1.87 (m,2H, CH2), 1.70 (m, 2H, CH2), 1.25-1.03 (m, 7H, CH and CH2). 13C NMR (CDCl3):δ 58.49 (CH),33.25 (CH3), 32.84 (CH2), 26.06 (CH2), 24.94 (CH2). IR (DCM): 3299, 3021, 2905,2871, 1489, 1312, 871, 775 cm-1. Mass Spec MS (EI) calcd for C7H15N: (M)+ 113, found:113 [1].
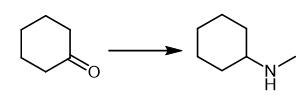
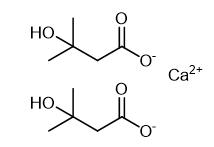
Fig. 3 The synthetic method 2 of N-Methylcyclohexylamine.
Prepare the concentration of ketone (10 mM), amine (25 mM), glucose (50 mM), GDH CDX-901 (0.3 mg/mL), NADP+ (0.4 mM), 100 μL imine reductase 66 enzyme in tris buffer (100 mM, pH 9.0) in total volume 500 μL. Incubate the reaction at 25°C, 250 rpm for 24 hours. Basify the reaction to pH 12 with add of NaOH (10 M, 40 μL) and CH2Cl2 (1 mL). Vortex the mixture and separate the organic layer. Dry the layer over MgSO4 prior to obtain the product [2].
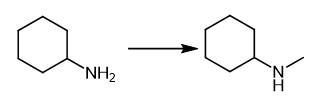
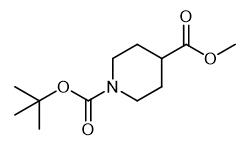
Fig. 4 The synthetic method 3 of N-Methylcyclohexylamine.
Benzylamine (0.068 mL, 0.63 mmol) was dissolved in dry DCM (3 mL), followed by the addition of Et3N (0.19 mL, 1.9 mmol) and 2-nitrobenzenesulfonyl chloride (0.139 g, 0.63 mmol). The mixture was stirred at room temperature for 6 h. The mixture was filtered; DCM (6 mL) was added, and Amberlite IRA-67 (1.0 g, 2 mmol, previously washed with H2O, MeOH, THF, and DCM) was added to the solution. The mixture shaken for 2 h. The resin was filtered off, and the solvent was evaporated under vacuum. Sulfonamide 3 (0.18 g, 98% yield) which was analyzed by 1H NMR and HPLC-MS analysis. The crude was dissolved into dry DMF (5 mL); to this solution, Cs2CO3 (0.2 g, 0.616 mmol) and MeI (0.088 mL, 1.39 mmol) were added, and the mixture stirred at room temperature for 12 h. The mixture was filtered; the solvent was evaporated, and the residue was dissolved into dry CHCl3. After 1 h of shaking in CHCl3, the solution was filtered through a sintered glass disc (maximum pore size of 16-40 μm), and the solvent was evaporated. Compound 4 (0.18 g, 95% yield) which was analyzed by 1H NMR and HPLCMS analysis. The crude was dissolved into dry THF (5 mL), and to this solution, Cs2CO3 (0.38 g, 1.2 mmol) was added, followed by PS-thiophenol resin (0.4 g of a 1.41 mmol/g loading resin, 0.56 mmol). The mixture was shaken for 12 h at room temperature. The solid was filtered off, and Cs2CO3 (0.38 g, 1.2 mmol) and PS-Thiophenol resin (0.4 g of a 1.41 mmol/g loading resin, 0.56 mmol) were added to the solution. The mixture was shaken for 12 h and filtered, and then, the solvent evaporated [3].
Application
Excess thermodynamic and spectroscopic study
Excess volume and sound speed data for three ternary mixtures, containing N-methylcyclohexylamine and bromobenzene as common components and 1-alkanols such as 1-propanol, 1-butanol, and 1-pentanol as third component, were measured at 303.15 K over the entire composition range. The experimental speeds of sound were used to compute isentropic compressibility and deviation in isentropic compressibility. The excess volume data of all mixtures were compared with data obtained from predictive expressions due to Redlich-Kister, Kohler, Tsao-Smith, and Hwang et al.'s equations. Further, the experimental results were analyzed in terms of molecular interactions between component molecules with FT-IR spectrum. Excess volume and speed of sound measured at 303.15 K for three ternary mixtures containing N-methylcyclohexylamine (1), bromobenzene (2), and 1-alcohols (3). Results were used to calculate derived thermodynamic properties and analyzed in terms of intermolecular interactions between component molecules [4].
Excess volume and isentropic compressibility study
Excess volume (V-123(E)) and speed of sound data for the ternary mixtures were reported at 303.15K for the ternary mixtures containing N-methylcyclohexylamine and chlorobenzene as common components and 1-alkanols namely 1-propanol, 1-butanol or 1-pentanol were chosen as non-common components. The experimental data of speed of sound (u) and density (rho(mix)(123)) of mixture were used to compute isentropic compressibility (k(s)(123)) and the quantity (Delta k(s)(123)), the difference between measured value and that of computed from the constituent binary data. Further, the experimental ternary excess volume data were compared with predictive relations. The measured data were analysed on the basis of intermolecular interactions between component molecules and also in terms of constituent binary data [5].
Volumetric, acoustic and FT-IR spectroscopic study
Excess volume (V-123(E)) and speed of sound (u(123)) data for three ternary mixtures, containing N-methylcyclohexylamine and nitrobenzene as common components and 1-alkanols, namely, 1-propanol, 1-butanol or 1-pentanol as non-common component were reported at 303.15 K. The experimental speeds of sound data of the mixtures were used to calculate the deviation in isentropic compressibility (Delta k(s123)). Further, experimental ternary excess volume data were compared with predictive relations in terms of Redlich-Kister, Kohler, Tsao-Smith and Hwang equations. Experimental results were analyzed on the basis of intermolecular interactions between component molecules with the help of FT-IR spectrum and also in terms of constituent binary mixtures [6].
References
[1] Chatterjee B, Kalsi D, Kaithal A, et al. One-pot dual catalysis for the hydrogenation of heteroarenes and arenes[J]. Catalysis Science & Technology, 2020, 10(15): 5163-5170.
[2] France S P, Howard R M, Steflik J, et al. Identification of novel bacterial members of the imine reductase enzyme family that perform reductive amination[J]. ChemCatChem, 2018, 10(3): 510-514
Lastest Price from N-Methylcyclohexylamine manufacturers

US $10.00/kg2025-04-21
- CAS:
- 100-60-7
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 100 mt

US $1190.00/kg2025-04-21
- CAS:
- 100-60-7
- Min. Order:
- 850kg
- Purity:
- 98%
- Supply Ability:
- 20 tons