Synthesis and Application of N-Methylbenzamide
General description
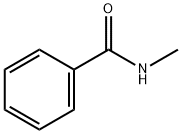
N-Methylbenzamide, a pale yellow or white solid with a burning point of 74°C, is an important pesticide intermediate. N-Methylbenzamide is a potent phosphodiesterase 10A (PDE10A) inhibitor. N-Methylbenzamide has anticancer activity.
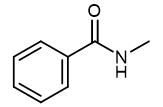
Fig. 1 The structure of N-Methylbenzamide.
Synthetic routes
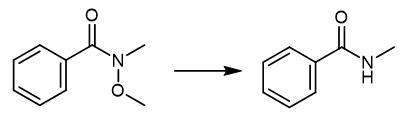
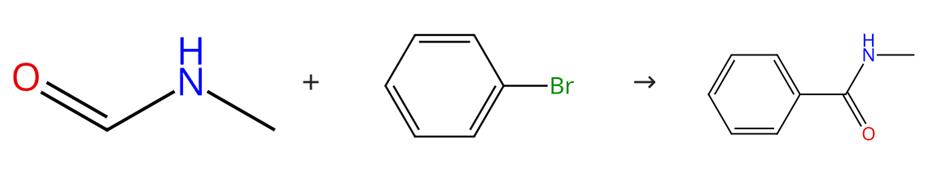
Fig. 2 The synthetic method 1 of N-Methylbenzamide.
Weigh N-methoxy-N-methylbenzamide (45 mg, 0.30 mmol, 1.0 equiv.), 2, 4, 5, 6-tetra (9H-carbazol-9-yl) isophthalonitrile (5 mol%) and LiBF4 (2.0 equiv.) in round bottom flask. Seal the flask, evacuate and purge with argon. Add degassed MeCN (0.1 M) and diisopropylethylamine (1.0 equiv.). Stir the reaction mixture for 16 h under light irradiation (402 nm). Remove the solvent in vacuo. Purify the crude residue by column chromatography (60% EtOAc in n-pentane). 1H NMR (400 MHz, DMSO-d6): δ (ppm) = 8.42 (s, 1H), 7.86 .-. 7.79 (m, 2H), 7.54 .-. 7.48 (m, 1H), 7.48 .-. 7.42 (m, 2H), 2.78 (d, J= 4.6 Hz, 3H) HRMS exact mass calculated for [M+Na]+(C8H9NONa+): 158.0576; found 158.0574 [1].
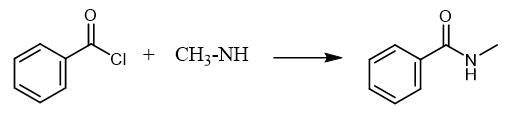
Fig. 3 The synthetic method 2 of N-Methylbenzamide.
Add benzoic acid (977 mg, 8 mmol), dry DCM (20 mL) and catalytic amount of DMF in a 100 mL round-bottom flask. Cool the reaction mixture to 0°C and stir for 5 minutes. Add (COCl)2 (0.89 mL, 1.3 equiv.) dropwise to the reaction mixture and stir at room temperature for 4 hours. Concentrate the resulting mixture under reduced pressure to obtain acid chloride. Add catalytic amount of 4-dimethylaminopyridine, dry DCM (15 mL), MeNH2 (2 (M) in THF) (5.19 mL, 1.3 equiv.) and Et3N (1.56 mL, 1.4 equiv.) to the mixture in a 100 mL round-bottom flask. Cool the reaction mixture to 0°C. Add acid chloride (1.0 equiv.) dropwise at 0°C. Stir the reaction mixture at room temperature for 12 hours. Add water (40 mL) and seperate the organic layer. Extract the aqueous layer with DCM (3 x 30 mL). Wash the combined organic layer with saturated aqueous NaHCO3 (30 mL) solution followed by water (30 mL). Dry the organic layer over Na2SO4 and concentrated under reduced pressure. Purify the crude mass by silica gel column chromatography (20% ethyl acetate in hexane as eluent) to obtain N-methylbenzamide [2].
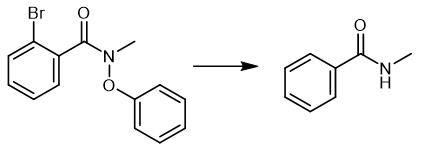
Fig. 4 The synthetic method 3 of N-Methylbenzamide.
Charge a 10 mL crimp vial, equipped with a magnetic stirring bar with methylene blue trihydrate (3.7 mg, 10 mol%), sodium dodecyl laurate (72 mg, 0.25 mmol), 2-bromo-N-benzyl-N-methylbenzamide (30.4 mg, 0.1 mmol) and distilled water (5 mL). Degas the resulting mixture via five pump-argon cycles. Add n-BuNH2 (0.30 mmol) to the reaction mixture under argon. Degas the mixture again via two short pump-argon cycles. Irradiate the reaction mixture with 7 W 455 nm LEDs through the plane bottom side. Stir the reaction mixture intensely for 20 h. Maintain the temperature at 40°C to 42°C by cooling with the built-in cooling fan. Open the vial. Transfer the crude reaction mixture to a separatory funnel. Add saturated solution of K2CO3 (5 mL) and brine (20 mL) to the reaction mixture. Extract the mixture with AcOEt (3 x 20 mL). Wash the combined organic fractions with fresh brine. Dry the combined organic fractions over MgSO4. Filter the combined organic fractions. Concentrate the filtrate in vacuo. Purify the crude product by flash column chromatography on silica gel (AcOEt:n-hexane 20%) to obtain N-methylbenzamide [3].
Application
Preparation of Dinuclear organoplatinum(II) complexes
The preparation and characterization of cationic, dinuclear complexes of the type trans-[Pt(PPh3)(2)(sigma-C6H4NHMe)-mu-L-Pt(PPh3)(2)(sigma-C6H4NHMe)](OTf)(2) (L = 4,4'-bipyridine, 4,7-phenanthroline, 4,4'-dipyrazolylmethane, and 1,1'-diphenyl-4,4'-dipyrazolylmethane; OTf = trifluoromethanesulfonate (triflate)) containing two C-3-N-methylbenzamide ligands are described. The key structural feature of the cationic complexes is the presence of two convergent amide groups that may allow for charge-assisted, H-bonding interactions in solution with suitable heteroaromatic guest molecules such as nucleobases [4].
As phosphodiesterase 10A (PDE10A) inhibitors
PDE10A is a recently identified phosphodiesterase with a quite remarkable localization since the protein is abundant only in brain tissue. Based on this unique localization, research has focused extensively on using PDE10A modulators as a novel therapeutic approach for dysfunction in the basal ganglia circuit including Parkinson's disease, Huntington's disease, schizophrenia, addiction and obsessive compulsive disorder. Medicinal chemistry efforts identified the N-methyl-N-[4-(quinolin-2-ylmethoxy)-phenyl]-isonicotinamide (8) as a nanomolar PDE10A inhibitor. A subsequent Lead-optimization program identified analogous N-methylanilides and their corresponding N-methylbenzamides (29) as potent PDE10A inhibitors, concurrently some interesting and unexpected binding modes were identified [5].
References
[1] Soika J, McLaughlin C, Nevesely T, et al. Organophotocatalytic N–O Bond Cleavage of Weinreb Amides: Mechanism-Guided Evolution of a PET to ConPET Plat-form[J]. 2022.
[2] Hoque M E, Bisht R, Unnikrishnan A, et al. Iridium‐Catalyzed Ligand‐Controlled Remote para‐Selective C? H Activation and Borylation of Twisted Aromatic Amides[J]. Angewandte Chemie, 2022: e202203539.
[3] Cybularczyk-Cecotka M, Predygier J, Crespi S, et al. Photocatalysis in Aqueous Micellar Media Enables Divergent C–H Arylation and N-Dealkylation of Benzamides[J]. ACS Catalysis, 2022, 12(6): 3543-3549.
[4] Crisp M G, Rendina L M. Dinuclear organoplatinum (II) complexes containing N-methylbenzamide[J]. Canadian Journal of Chemistry, 2009, 87(1): 212-216.
[5] Kilburn J P, Kehler J, Langg?rd M, et al. N-Methylanilide and N-methylbenzamide derivatives as phosphodiesterase 10A (PDE10A) inhibitors[J]. Bioorganic & medicinal chemistry, 2013, 21(19): 6053-6062.
You may like
Related articles And Qustion
See also
Lastest Price from N-Methylbenzamide manufacturers

US $0.00-0.00/KG2025-07-22
- CAS:
- 613-93-4
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 2000

US $100.00/kg2025-04-21
- CAS:
- 613-93-4
- Min. Order:
- 1kg
- Purity:
- 99% Purity (What/sapp: +86 18145728414)
- Supply Ability:
- 1000 Tons/Month