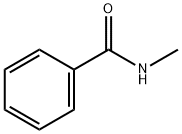
Synthesis and Applications of N-Methylbenzamide
General description
N-methylbenzamide, a chemical compound represented by the formula C8H9NO, is an amide derived from benzamide. This organic compound has garnered interest across various scientific and industrial fields due to its unique chemical properties and versatility.
Synthesis
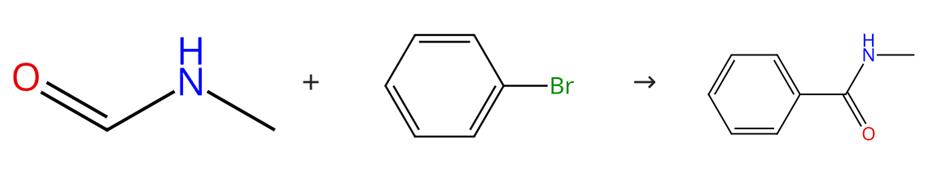
Fig. 1 The synthesis route ofN-methyl benzamide
Scheme 1:Combine Ni(OAc)2·4H2O (0.03 mmol), phosphite (19.5 mg, 0.03 mmol) and aryl bromide (3.0 mmol) with NaOMe (324 mg, 6.0 mmol) in a small roundbottom flask.Add 1-bromobenzene (471.0 mg, 3.00 mmol) andN-methylformamide (531.6 mg, 9.0 mmol) to the mixture.Seal the flask with a septum. Place the resulting mixture in an oil bath at 110 °C for 10 hours. Pour the reaction mixture into 20 ml of saturated aqueous ammonium chloride. Extract the mixture (3 x 20 ml) with Et2O. Wash the combined ether extract with brine (60 ml). Dry the combined ether extract over MgSO4.Filter the combined ether extract. Remove the solvent under vacuum. Purify the resulting crude product by flash chromatography on silica gel eluting with 70% ethyl acetate in hexane. Recrystallize the product from Et2O. The synthesis route is shown in Fig. 1[1].
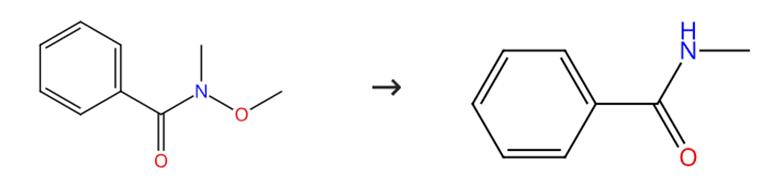
Fig. 2 The synthesis route ofN-methyl benzamide
Scheme 2:Treat a 0 °C solution of diisopropylamine (147 μL, 1.05 mmol) in tetrahydrofuran (4 mL) with nBuLi (1.0 mmol). Stir the mixture for 15 minutes. Add tributyltin hydride (255 μL, 0.96 mmol) to the mixture. Stir the mixture for an additional 15 minutes. Cool the solution of Bu3SnLi thus prepared to -40 °C. Treat the mixture with N, N-dimethylbenzamide (0.8 mmol). Stir the reaction mixture at -40 °C for 4 hours. Quench the mixture by adding of pH 7.0 phosphate buffer solution (2 mL). Partition the mixture between pH 7.0 phosphate buffer solution and CH2Cl2. Extract the aqueous layer with CH2Cl2 (10 mL). Wash the combined organic phase with brine (15 mL). Dry the combined organic phase. Filter the combined organic phase. Concentrate the combined organic phase under reduced pressure. Purify the residue by column chromatography (SiO2, EtOAc: hexane 1:15). The synthesis route is shown in Fig. 2[2].
Applications
In the pharmaceutical industry, N-methylbenzamide serves as a pivotal intermediate in the synthesis of a wide range of drugs and active pharmaceutical ingredients (APIs). Its chemical structure enables it to undergo various chemical reactions, making it a versatile precursor in the manufacture of analgesics, antipyretics, and anti-inflammatory drugs.
Beyond its pharmaceutical applications, N-methylbenzamide is also utilized in organic chemistry as a reagent and catalyst for a variety of synthetic processes. Its unique chemical properties facilitate the synthesis of complex organic molecules through condensation reactions, acylation, and as a component in the preparation of amides and esters. Its effectiveness as a catalyst in these reactions often results in higher yields and more efficient synthesis processes, making it a valuable asset in organic chemistry laboratories and industrial production.
In material science, N-methylbenzamide finds use in the development and modification of polymers and resins. Its chemical structure can enhance the physical properties of materials, such as thermal stability, flexibility, and resistance to chemicals. This makes it an important component in the creation of high-performance materials used in various industries, including automotive, aerospace, and electronics. The incorporation of N-methylbenzamide into polymers can lead to materials with improved performance characteristics, catering to the specific needs of these advanced applications.
Additionally, N-methylbenzamide is recognized for its role as a high-purity solvent in analytical and synthetic chemistry. Its ability to dissolve a wide range of organic compounds makes it an excellent choice for facilitating chemical reactions and processes. In analytical chemistry, it is used as a solvent in spectroscopy and chromatography, providing a medium for the analysis of complex mixtures. Its non-toxic and stable nature under various conditions further enhances its suitability as a solvent in sensitive chemical analyses and reactions.
The versatility and utility of N-methylbenzamide across multiple fields highlight its importance in scientific research and industrial applications. Its role in pharmaceutical synthesis contributes to the development of new and effective medications, while its applications in organic synthesis enhance the efficiency and outcomes of chemical reactions. Furthermore, its contribution to material science leads to the creation of advanced materials with superior properties, and its use as a solvent supports crucial analytical and synthetic processes.
References
[1]Jo, Youngshin; et al.The Scope and Limitation of Nickel-Catalyzed Aminocarbonylation of Aryl Bromides from Formamide Derivatives. Journal of Organic Chemistry (2009), 74(16), 6358-6361.
[2]Paleo, M. Rita; et al.Stanna-Brook Rearrangement of Carboxylic Acid Derivatives. Synthetic Utility and Mechanistic Studies.Organic Letters (2004), 6(6), 1061-1063.
Related articles And Qustion
See also
Lastest Price from N-Methylbenzamide manufacturers

US $0.00-0.00/KG2025-07-22
- CAS:
- 613-93-4
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 2000

US $100.00/kg2025-04-21
- CAS:
- 613-93-4
- Min. Order:
- 1kg
- Purity:
- 99% Purity (What/sapp: +86 18145728414)
- Supply Ability:
- 1000 Tons/Month