Synthesis and Application of Diethylamino hydroxybenzoyl hexyl benzoate
General description
Diethylamino hydroxybenzoyl hexyl benzoate (DHHB), also known as 4-methylphenyl thiourea and p-methylphenyl thiourea, is an ester compound with a relative molecular weight of 397.5072. DHHB absorbs UV-A radiation with a peak at 354 nm. The molar absorption coefficient of Diethylamino hydroxybenzoyl hexyl benzoate in EtOH at 25°C is obtained to be 39 000 mol/dm3/cm at 354 nm.
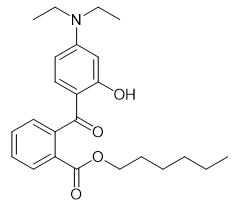
Fig. 1 The structure of Diethylamino hydroxybenzoyl hexyl benzoate.
Synthetic routes
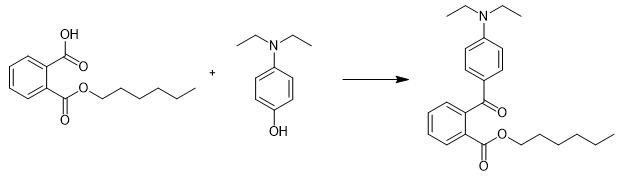
Fig. 2 The synthetic step 1 of Diethylamino hydroxybenzoyl hexyl benzoate.
Add 3.5 L of methylene chloride, 355 g (1.35 mol) of 2-hexanoxycarbonylbenzoic acid (VII), 17.17 g (0.139 mol) of 4-dimethylaminopyridine, and 333.8 g (2.02 mol) of 3-diethylaminophenol (X) to a 5-L four-necked round flask, immersed in a thermal bath, equipped with a mechanical stirrer, thermometer, dropping funnel, and cooler connected to a calcium chloride tube. Cool the solution to to 20-25 °C. Add 321.6 (1.55 mol) of dicyclohexylcarbodiimide slowly . Stir the resulting mixture for 4 hours at 20-25 °C. Monitor the total transformation of 2-hexanoxycarbonylbenzoic acid (VII) by thin-layer chromatography (Cl3CH:AcOEt; 80:20). Filter the formed dicyclohexylurea. Wash the cake with 530 mL of methylene chloride. Wash the methylene chloride solution with 1.79 L of 2N NaOH aqueous solution. Concentrate the mixture under reduced pressure. Dissolve the resulting oil in n-heptane. Wash with 1.2 L of 4N AcOH aqueous solution, with 300 mL of 20% sodium carbonate aqueous solution, and with 600 mL of water. Discolor the solution by stirring with active carbon. Filter the discolored solution. Concentrate the mixture under reduced pressure to obtain the product. 1H NMR (CDCl3, TMS, 300 MHz) δ 7.91 (m, 1H, HAr), 7.75 (m, 1H, HAr), 7.58 (m, 2H, HAr), 7.20 (m, 1H, HAr), 6.53 (m, 3H, HAr), 4.31 (t, 2H, -CO2CH2-), 3.35 (q, 4H, CH2(Et)), 1.71 (m, 2H, -CH2-), 1.29 (m, 6H, -CH2-CH2-CH2-), 1.18 (t, 6H, CH3(Et)), 0.86 (t, 3H, -CH3(Hex)) 11B NMR (CDCl3, TMS, 75 MHz) δ 167.4, 165.8, 152.1, 148.9, 132.7, 131.5, 131.3, 130.8, 129.7, 129.3, 128.7, 109.2, 107.8, 104.3, 66.0, 44.5, 31.5, 28.6, 25.6, 22.6, 14.1, 12.6 [1].
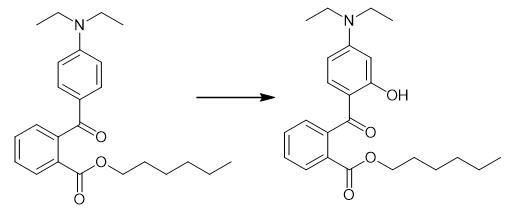
Fig. 3 The synthetic step 2 of Diethylamino hydroxybenzoyl hexyl benzoate.
Expose 3 mL of a 0.018 mg/mL solution of 3-diethylaminophenyl 2-hexanoxycarbonylbenzoate in methanol to UV-B radiation in a Luzchem LZC-4 photoreactor equipped with 16 broad band UV-B lamps (LES-UVB-01) at 35 °C to obtain hexyl 2-(4-diethylamino-2-hydroxybenzoyl)benzoate [1].
As Sunscreens
NP TLC Analysis
A simple, rapid, and effective method for TLC separation of two EU-authorized UV filters octyl methoxycinnamate (OMC) and diethylamino hydroxybenzoyl hexyl benzoate (DHHB) on silica gel 60 is proposed. Separation conditions were optimized and cyclohexane-diethyl ether-acetone 15:1:2 (v/v) was used as mobile phase. Quantification of both filters on the same chromatographic plates was achieved by densitometric scanning in absorption-reflectance mode at 300 and 360 nm, respectively. The method enabled separation of other UV-absorbing ingredients commonly found in cosmetic sunscreen preparations, for example parabens or ethylhexyltriazone (ET). Good quality, linear calibration plots were generated over the range 200-2000 ng per spot both for OMC and DHHB. The method was validated [2].
Degradation
Steady-state and transient-state photolysis experiments were conducted to investigate the degradation of organic ultraviolet filter diethylamino hydroxybenzoyl hexyl benzoate (DHHB) in the aqueous solution by UV/H2O2. Results showed that the obvious degradation of DHHB was not observed under UV irradiation (lambda= 254 nm), and the DHHB degradation was conducted due to the oxidation by hydroxyl radical (HO center dot). While the H2O2 concentration was between 0.05 and 0.10 mol L-1, the highest DHHB degradation efficiency was obtained. The lower solution pH favored the transformation of DHHB, and the coexisting Cl-and NO3- ions slightly enhanced the conversion. The degradation of DHHB by HO center dot followed a pseudo-first-order kinetic model with different initial DHHB concentrations. By intermediate products during DHHB oxidation and laser flash photolysis spectra analysis, a primary degradation pathway was proposed [3].
Detection method
The aim of the study was the validation of a high-performance liquid chromatography (HPLC) method for the simultaneous and quantitative determination of twelve commonly used organic UV-filters (phenylbenzimidazole sulfonic acid, benzophenone-3, isoamyl p-methoxycinnamate, diethylamino hydroxybenzoyl hexyl benzoate, octocrylene, ethylhexyl methoxycinnamate, ethylhexyl salicylate, butyl methoxydibenzoylmethane, diethylhexyl butamido triazone, ethylhexyl triazone, methylene bis-benzotriazolyl tetramethylbutylphenol and bis-ethylhexyloxyphenol methoxyphenyl triazine) contained in suncare products. The separation and quantitative determination was performed in < 30 min, using a Symmetry Shield (R) C18 (5 mu m) column from Waters and a mobile phase (gradient mode) consisting of ethanol and acidified water. UV measurements were carried out at multi-wavelengths, according to the absorption of the analytes [4].
References
[1] Sallares Rosell J, Nonell S, Marquillas Olondriz F, et al. 3-Dialkylaminophenyl 2-alkoxycarbonylbenzoic compounds as precursor ultraviolet absorbents for use as sunscreens[P]. PCT Int. Appl., 2010125092, 2010.
[2] Sobanska A W, Brzezinska E. Simultaneous NP TLC analysis of the sunscreens diethylamino hydroxybenzoyl hexyl benzoate and octyl methoxycinnamate[J]. JPC–Journal of Planar Chromatography–Modern TLC, 2011, 24(3): 227-231.
[3] Gong P, Yuan H, Zhai P, et al. Degradation of organic ultraviolet filter diethylamino hydroxybenzoyl hexyl benzoate in aqueous solution by UV/H2O2[J]. Environmental Science and Pollution Research, 2015, 22(13): 10189-10195.
[4] Nyeborg M, Pissavini M, Lemasson Y, Doucet O. Validation of HPLC method for the simultaneous and quantitative determination of 12 UV-filters in cosmetics[J]. International Journal of Cosmetic Science, 2010, 32(1): 47-53.
Related articles And Qustion
See also
Lastest Price from Diethylamino hydroxybenzoyl hexyl benzoate manufacturers

US $0.00-0.00/KG2025-07-05
- CAS:
- 302776-68-7
- Min. Order:
- 1KG
- Purity:
- 98.0%
- Supply Ability:
- 10000KGS

US $1.00-4.00/KG2025-05-19
- CAS:
- 302776-68-7
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 1KG 100K