Crystal structure and THz-TDS investigation of gallic acid monohydrate
Introduction
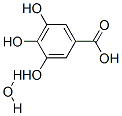
Gallic acid (3,4,5-trihydroxybenzoic acid) and gallic acid monohydrate (Figure 1), as an intermediate component of plant metabolism from the hydrolysis of tannins, is distributed in plants and foods widely. It has been associated with a variety of biological actions including antioxidant, antibacterial, antiviral and antimicrobial. Gallic acid has been reported to have five monohydrates and three anhydrous forms by Braumet al.. Among them, two kinds of gallic acid monohydrated forms, monohydrated form I (MH-I) and monohydrated form IV (MH-IV),were already reported in the Cambridge Structural Database. MH-I of gallic acid was found to be relatively stable at ambient condition, and it is the commercial crystalline form as well. Recently research about monohydrated form II and III (MH-II, MH-III) has also been published,as showing remarkable hydrate polymorphism. Monohydratedform V (MH-V), the least stable form, was prepared by evaporating gallic acid from 2-butanol and it would transform to either one of the three anhydrous forms or a hydrate (MH-I, MH-III, MH-IV). Anhydrous gallic acid is also showing polymorphic effect, though only solvent free structure has been reported as anhydrate form II (AH-II), and the other two forms (AH-I and AH-III) are metastable polymorphs. Various crystalline forms would represent diverse physical properties, such as density, solubility, hardness, melting point, bioavailability etc. And consequently can profoundly influence the manufacturing process,long-term stability and performance of drug products.[1]

Structure and dehydration dynamic of gallic acid monohydrate
The dehydration process of gallic acid monohydrate was carried out by heating method and characterized using Raman spectroscopic technique. Density functional theory calculation with B3LYP function is applied to simulate optimized structures and vibrational frequencies of anhydrous gallic acid and its corresponding monohydrated form. Different vibrational modes are assigned by comparison between experimental and theoretical Raman spectra of above two polymorphs. Raman spectra show that vibrational modes of the monohydrate are distinctively different from those of anhydrous one. Meanwhile, the dynamic information about dehydration process of gallic acid monohydrate could also be observed and monitored directly with the help of Raman spectral analysis. The decay rate of the characteristic band from gallic acid monohydrate and the growth rate of anhydrous one are pretty consistent with each other. It indicates that there is no intermediate present during the dehydration process of gallic acid monohydrate. The results could offer us benchmark works for identifying both anhydrous and hydrated pharmaceutical compounds, characterizing their corresponding molecular conformation within various crystalline forms, and also providing useful information about the process of dehydration dynamic at the microscopic molecular level.[1]
Crystal structure of Gallic acid monohydrate
In the crystal structure of the title compound, 3,4,5-trihydroxybenzoic acid monohydrate, C7H6O5·H2O, the gallic acid molecule has an intramolecular hydrogen bond involving a pair of hydroxyl groups, and it is also linked to a water molecule by a three-centre (bifurcated) OW-H┅O hydrogen bond. The packing of the molecules is stabilized by intermolecular O-H┅O and C-H┅┅O hydrogen bonds.
Gallic acid monohydrate is essentially planar. The mean deviation of the benzene ring is 0.0028 Å and its dihedral angle with the plane of the carboxyl group is 2.9º. The bond distances are all normal.Within the asymmetric unit, there is an intramolecular hydrogen bond between O1 and O2. The water molecule is linked to the gallic acid by a three-centre (bifurcated) donor hydrogen bond to two acceptor hydroxyl groups. These hydrogen bonds form two five-membered hydrogen-bonded rings. The intermolecular hydrogen bondsO3-H┅OW, O2-H┅OW and OW-HOWA┅O2 link the asymmetric unit at the junction where the water molecules are spirally distributed, to form a channel running parallel to the a axis. The intermolecular hydrogen bond O4-H┅O5 linksthe two adjacent channels and results in extended ‘wavy’ sheets parallel to the (200) plane. Adjacent sheets are linked by hydrogen bond O1-H┅O5 and a weak C-H┅O hydrogen bond between C6 and O1 to form a supramolecular assembly. [2]
Terahertz spectroscopic investigation of gallic acid monohydrate
As a novel technique, terahertz (THz) spectroscopy is regarded as an advanced approach to study new aspects of molecular structure and distinguish polymorph pharmaceutical, especially useful to biochemical molecules which contain rich hydrogen-bond interactions.Walther et al. utilized the terahertz absorption spectra which involve fingerprints of the molecular structure to identify benzoic acid and its derivates.In the present work, gallic acid and gallic acid monohydrate were studied by THz-TDS in the spectral range of 0.5 to 4.5 terahertz. Solid-state DFT calculations based on the samples crystalline structures were performed to simulate the vibrational modes of gallic acid and gallic acid monohydrate molecules to understand the experimental terahertz spectra better.
The low-frequency spectra of gallic acid (GA) and gallic acid monohydrate were investigated by terahertz time-domain spectroscopy (THz-TDS) in the range of 0.5 to 4.5THz. The dehydration process of gallic acid monohydrate was monitored on-line. The kinetic mechanism of the dehydration process was analyzed depending on the terahertz spectral change at different temperatures. The results indicate that the diffusion of water molecule dominates the speed of the entire dehydration process. Solid-state density functional theory (DFT) calculations of the vibrational modes of both gallic acid and gallic acid monohydrate were performed based on their crystalline structures for better interpreting the experimental terahertz spectra. The results demonstrate that the characterized features of gallic acid mainly originate from the collective vibrations of molecules. And the interactions between gallic acid and water molecules are responsible for THz fingerprint of gallic acid monohydrate. Multi-techniques including differential scanning calorimetry and thermogravimetry (DSC-TG) and powder X-ray diffraction (PXRD) were also carried out to further investigate gallic acid and gallic acid monohydrate.[3]
References
[1]Cai Q, Xue J, Wang Q, Du Y. Investigation into structure and dehydration dynamic of gallic acid monohydrate: A Raman spectroscopic study. Spectrochim Acta A Mol Biomol Spectrosc. 2018;201:128-133. doi:10.1016/j.saa.2018.05.002
[2]Jiang RW, Ming DS, But PP, Mak TC. Gallic acid monohydrate. Acta Crystallogr C. 2000;56 ( Pt 5):594-595. doi:10.1107/S0108270100001827
[3]Zhang B, Li S, Wang C, et al. Terahertz spectroscopic investigation of gallic acid and its monohydrate. Spectrochim Acta A Mol Biomol Spectrosc. 2018;190:40-46. doi:10.1016/j.saa.2017.09.004
Lastest Price from Gallic acid monohydrate manufacturers

US $0.00/kg2025-05-09
- CAS:
- 5995-86-8
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 1000kg

US $0.00-0.00/KG2025-04-15
- CAS:
- 5995-86-8
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 500000kg