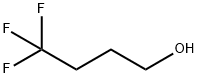
Synthesis and Application of 4,4,4-Trifluoro-1-Butanol
General description
4,4,4-Trifluoro-1-Butanol is an important fluorinated organic intermediate widely used in semiconductor, liquid crystal and pharmaceutical fields. As a semiconductor intermediate, it is mainly used to synthesize TCNQ derivatives. As an intermediate of liquid crystal materials, it is mainly used to synthesize biphenyl liquid crystal materials containing fluoroalkyl ethers. It is used in the pharmaceutical field, mainly for the synthesis of inhibitors of monoamine oxidase B, lactones and lactam inhibitors of central region, anti-schizophrenia drugs, immune agents, etc.
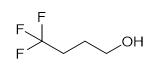
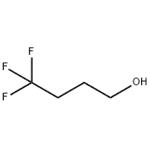
Fig. 1 The structure of 4,4, 4-Trifluoro-1-Butanol.
Synthetic routes

Fig. 2 The synthetic step 1 of 4,4, 4-Trifluoro-1-Butanol.
The magnetic stirrer and thermometer were added to the 500 mL three-mouth bottle, and 5.2 g p-toluenesulfonic acid (0.03 mol), 94.5 g 3-chloropropanol (l.0 mol), 200 mL methylene chloride, 16.2g 3, 4_ dihydropyran (1.5 mol) were added to the bottle. The reaction was stirred at room temperature 20-25 °C for l h. After the reaction solution was tested by gas chromatography for 3-chloropropanol, l00 mL water was added to the system, and the phase was separated after stirring for 5 min. The organic phase was washed with l00 mL saturated salt water, dried with 10 g anhydrous sodium sulfate, filtered and concentrated to remove the solvent. Raw 182.5g. After vacuum distillation, 110-115C fraction (vacuum degree 30mbar) was collected to obtain colorless transparent liquid 164.6 g, which was 2- (3-chloropropoxy) tetrahydro-2h-pyron, with GC content of 98.2 % and yield of 91.13% [1].
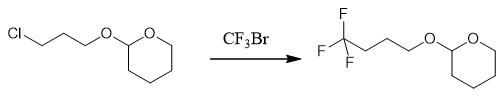
Fig. 3 The synthetic step 2 of 4,4, 4-Trifluoro-1-Butanol.
The dry l000 mL three-mouth flask was vacuumed and replaced with N2 for 3 times. 3.7 g dry activated alumina for chromatography (200-300 mesh) was added under the atmosphere of N2 and heated to 120°C. 0.37 g (0.016 mol) was quickly added under fierce stirring. Forming a grayish black high surface metallic sodium coating. After the temperature drops to room temperature, 600 mL anhydrous tetrahydrofuran is added and placed in the low temperature tank to cool to -10°C. 142.9 g 2- (3-chloropropoxy) tetrahydro-2H-peyran (0.8 mol) is dropped in, and 131.0 g trifluoromethane (0.88 mol) is poured in at the same time, with a molar ratio of 1:1:1. Control temperature _10 °C to 0°C. After feeding, continue stirring for 30 min. After the reaction solution was tested by gas chromatography for 2- (3-chloropropoxyl) tetrahydro-2H-pyran reaction, the catalyst was removed by pressure filtration under nitrogen atmosphere. Under nitrogen atmosphere, 100 mL water and 200 mL methylene chloride were slowly added to the mother liquor, stirred for 5 ~ l0 min, and the phase was separated after standing. The aqueous phase was extracted three times with methylene chloride. 100 mL each time. Combined with the organic phase, 20 g of anhydrous sodium sulfate was dried, filtered and concentrated to remove the solvent, yielding 136.1 g of crude product. After vacuum distillation, the 120-123 fraction (vacuum 50mbar) was collected to obtain 122.8 g colorless transparent liquid, which was 2- (4, 4, 4-trifluorobutanoxy) tetrahydro-2h-pyron, with 98.6% GC content and 72.34% yield [1].

Fig. 4 The synthetic step 3 of 4,4, 4-Trifluoro-1-Butanol.
300mL ethanol, 106.1 g 2- (4,4,4-trifluorobutanoxy) tetrahydro-2H-piyran (0.5 mol) and 2.5 g PPTs (0.01 mol) were added to the 500mL three-mouth flask, and the reaction was heated to 50°C for 1 h. After the reaction solution was detected by gas chromatography for 2- (4,4,4-trifluorobutanoxy) tetrahydro-2H-piran, 200 mL dichloromethane and 200 mL water were added to the system, stirred for 5-l0 min, and then stood for phase separation. The aqueous phase was extracted with dichloromethane three times, 150 mL each time. Combined with organic phase, 20 g anhydrous sodium sulfate was dried and filtered. The mother liquor was directly distilled, 100 ~ 130°C fraction was collected, and then the 64 ~ 66°C fraction (vacuum 55 mbar) was collected to obtain colorless transparent liquid 58.0 g, which was 4, 4, 4-trifluoro-1-butanol. The GC content was more than 99%, and the yield was 90.56% [1].
Application
Because of the high electronegativity of fluorine atoms,4,4 trifluoride 1 butanol has a strong hydrogen bond formation ability, and other chemicals will generate more stable compounds; Compared with non-fluorinated compounds, such fluorinated compounds have the characteristics of less dosage, low toxicity, high efficacy and strong metabolic ability in pharmaceutical and pesticide properties, and are widely used as drugs with stronger biological activity and higher selectivity. 4,4,4 trifluoride 1 butanol can be used in medicine to prepare lactones and lactam compounds (used as central nervous system inhibitors), immune agents, etc.; It can also be used to prepare liquid crystal materials and organic conductors, and has a broad prospect for development and utilization [2].
References
[1] Wang G, Peng Z, Liu B, et al. Preparation of 4,4,4-trifluoro-1-butanol involves carrying out addition reaction of 3-chloropropanol with 3,4-dihydropyran under action of catalyst, and cross-coupling halotrifluoromethane with 2-(3-chloropropoxy)tetrahydro-2H-pyran[P]. Faming Zhuanli Shengqing, 109336740, 2019.
[2] Du X, Cheng J. Preparing 4,4,4-trifluoro-1-butanol, comprises reacting ethyl trifluoroacetate with Grignard reagent to obtain 4-(benzyloxy)-1,1,1-trifluorobutan-2-one polymer, then performing reduction reaction of obtained product, and hydrolyzing[P]. Faming Zhuanli Shengqing, 112778089, 2021.
You may like
Lastest Price from 4,4,4-TRIFLUORO-1-BUTANOL manufacturers

US $0.00-0.00/KG2025-04-15
- CAS:
- 461-18-7
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 500000kg
US $0.00-0.00/kg2025-04-04
- CAS:
- 461-18-7
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1Ton