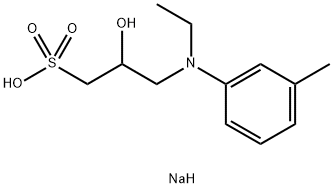
Application of Sodium 3-(N-ethyl-3-methylanilino)-2-hydroxypropanesulfonate
General description
Sodium 3-(N-ethyl-3-methylanilino)-2-hydroxypropanesulfonate (TOOS) is a highly soluble aniline derivative used for catalase spectrophotometric determination. For quantitative determination of uric acid (UA) concentration in human serum, plasma or urine only. Good water solubility, high sensitivity, strong stability. The new Trinder's reagent is stable enough to be used in both solution and test line detection system. In the presence of hydrogen peroxide and peroxidase, the novel Trinder's reagent forms a very stable purple or blue dye during the oxidative coupling reaction with 4-aminoantipyrine (4-AA) or 3-methylbenzothiazolium sulfoxide (MBTH).
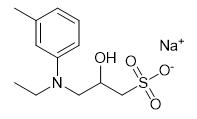
Fig. 1 The structure of Sodium 3-(N-ethyl-3-methylanilino)-2-hydroxypropanesulfonate.
Application
As chromogenic substrates
Nanozymes, as a new type of artificial enzyme, have recently become a research hotspot in the field of catalysis and biomedicine. However, the application of nanozyme is limited by catalytic activity changes of different substrates and low specificity. This work shows that citrate-capped platinum nanoparticles (Cit-PtNPs) exhibit stronger oxidase-like activity than other platinum nanozymes at different pH when 3-methyl-2-benzothiazolinonehydrazone hydrochloride (MBTH) and n-ethyl-n- (2-hydroxy-3-sulfopropyl)-m-toluidine sodium salt (TOOS) were used as chromogenic substrates. This phenomenon has important reference value for different nanozymes to choose chromogenic substrates in catalysis. In MBTH-TOOS chromogenic system, MBTH (-NH) radical is first produced during the reaction through catalytic oxidation of Cit-PtNPs, which reacts with TOOS to produce a colorless compound. The blue-purple quinoid dye was produced through the dismutation of the colorless compound. The catalytic mechanism of the oxidase-like activity of Cit-PtNPs is that two-electron reduction process and four-electron reduction process are simultaneously carried out in the catalytic process. Furthermore, to solve the problem of low specificity of metal nanozymes, protamine is designed as aggregation promoter of Cit-PtNPs and the specifichydrolysis substrate of trypsin. In this work, it can achieve one-step detection of trypsin by the boosting oxidase activity of Cit-PtNPs at pH8. The catalytic activity of Cit-PtNPs is proportional to the concentration of trypsin. The linear range for trypsin is 1.0-70.0 ngmL(-1) and the limit of detection is measured to be 0.6 ngmL(-1). This novel method has also been successfully applied to the detection of inhibitors and trypsin in urine samples [1].
A spectrophotometric method was developed and validated for choline in enteral nutrition. The method involves an enzymatic conversion of choline by choline oxidase to hydrogen peroxide, which reacts with 3-(N-ethyl-3-methylanilino)-2-hydroxypropanesulfonic acid (TOOS) and 4-aminoantipyrine in the presence of peroxidase to form a purple colored product with the optimal absorption at 554 nm. The test conditions were optimized for avoiding interference by any active ingredient other than choline in a commercial drug product (ELENTAL (R)). This method was validated for the determination of choline bitartrate in the ELENTAL (R), and demonstrated good specificity, linearity, accuracy and precision. The proposed method was successfully applied to assays of choline in commercial drug products (ELENTAL (R) and HEPAN ED (R)). The proposed method is simple, specific and useful for the determination of choline in enteral nutrition and supplements [2].
Determination of uric acid
In this work a new enzymatic method for the determination of uric acid in human serum has been developed. The method is based on the oxidative coupling reaction between the N-methyl-N-(4-aminophenyl)-3-methoxaniline (NCP) reagent and the hydrogen-donor reagent. N-ethyl-N-(2-hydroxy-3-sulfopropyl)-3-methylaniline (TOOS). in the system involving three enzymes: tin. case. peroxidase and ascorbate oxidase. Using this method uric acid could be determined in concentrations tip to 1.428 mmol/L, with a relative standard deviation of tip to 1.8 %. The effect of the medium pH and the NCP concentration on the linearity of the chromogen absorbance versus the uric acid concentration curve was investigated. The influence of the uricase activity on the maximum rate of uric acid oxidation AN as also examined. The use of the NCP reagent demonstrated a more precise and more sensitive determination of the uric acid compared to the determination with 4-aminoantipyrine (4-AA) as the coupling regent. The sensitivity of the method determined from the calibration curve was 0.71 absorbance units per mmol/L of uric acid. the limit of detection was LOD = 0.0035 mmol/L and the limit of quantification was LOQ = 0.0151 mmol/L of uric acid [3].
References
[1] Lin X, Zhu Z, Lin D, et al. Boosting the oxidase-like activity of platinum nanozyme in MBTH-TOOS chromogenic system for detection of trypsin and its inhibitor[J]. Talanta, 2021, 234: 122647.
[2] Sugiyama S, Kondo J, Okamoto M, et al. A spectrophotometric assay for choline in enteral nutrition[J]. Bunseki Kagaku= Journal of Japanese Society for Analytical Chemistry, 2014, 63(9): 743-749.
[3] Jeliki?-Stankov M D, ?ur?evi? P T, Stankov D. Determination of uric acid in human serum by an enzymatic method using N-methyl-N-(4-aminophenyl)-3-methoxyaniline reagent[J]. Journal of the Serbian Chemical Society, 2003, 68(8-9): 691-698.
You may like
See also
Lastest Price from Sodium 3-(N-ethyl-3-methylanilino)-2-hydroxypropanesulfonate manufacturers

US $0.00-0.00/KG2025-09-02
- CAS:
- 82692-93-1
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 3500kg/month

US $0.00/KG2025-08-30
- CAS:
- 82692-93-1
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 2000KG