Synthesis and Application of (3S)-3-[4-[(5-Bromo-2-chlorophenyl)methyl]phenoxy]tetrahydrofuran
General description
(3S)-3-[4-[(5-Bromo-2-chlorophenyl)methyl]phenoxy]tetrahydrofuran was used as a reactant to efficiently synthesize SGLT- 2 Epagliflozin inhibitors.
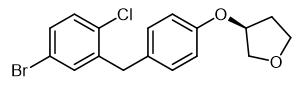
Fig. 1 The structure of (3S)-3-[4-[(5-Bromo-2-chlorophenyl)methyl]phenoxy]tetrahydrofuran.
Synthetic routes
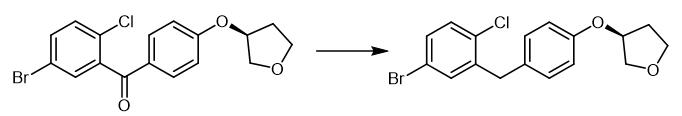
Fig. 2 The synthetic method 1 of (3S)-3-[4-[(5-Bromo-2-chlorophenyl)methyl]phenoxy]tetrahydrofuran.
To a mixture of AlCl3 (61.7 g, 0.463 mol) and (S)-(5-bromo-2-chlorophenyl)(4-((tetrahydrofuran-3-yl)oxy)phenyl)methanone (100 g, 0.231 mol) in toluene (450 mL) was added 1,1,3,3-tetramethyldisiloxane (40.4 g, 0.301 mol) over 1 hour at <20 °C. The mixture was held at 20-23 °C for an additional 1.5 hours and cooled to 0-5 °C. The cooled solution was slowly added to ice-water (400 mL) over 15 min and the layers separated. The organic was treated with 3N NaOH (400 mL) at room temperature for 16-24 hours and the layers were separated. The organic was concentrated to a low volume (~130 mL). After diluted with acetonitrile (450 mL), the solution was again concentrated to a low volume (~130 mL). To the residue was added acetonitrile (400 mL) followed by a slow addition of water (200 mL). The resulting mixture was cooled to 0-3 °C and held for 2 hours. The solids were collected by filtration and dried in oven to give product. (S)-3-(4-(2-chloro-5- bromobenzyl)phenoxy)tetrahydrofuran (2a). 92% as white crystals. 1H NMR (400 MHz, CDCl3) δ 7.27-7.24 (m, 2H), 7.07 (d, J = 8.4 Hz, 1H), 7.08 (d, J = 8.8 Hz, 2H), 6.79 (d, J = 8.8 Hz, 2H), 4.88 (m, 1H), 4.00-3.94 (m, 5H), 3.91-3.85 (m, 1H), 2.22-2.11 (m, 2H); 13C NMR (400 MHz, CDCl3) δ 156.1, 141.2, 133.6, 133.1, 130.9, 130.6, 130.1, 120.5, 115.5, 77.3, 73.2, 67.2, 38.2, 33.1; HRMS calculated for C17H20ClBrNO2 [M+NH4]+: 384.0380, Found: 384.0356. [1].
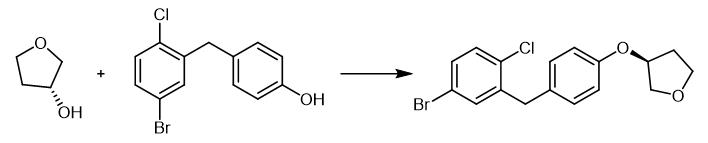
Fig. 3 The synthetic method 2 of (3S)-3-[4-[(5-Bromo-2-chlorophenyl)methyl]phenoxy]tetrahydrofuran.
Add diisopropyl azodicarboxylate dropwise to a suspension of (R) -THF-3-ol (5.6 g, 63.18 mmol) and triphenylphosphine (16.5 g, 63.18 mmol) in dry DCM (100 mL) at 0°C. Allow the temperature to reach room temperature and stir the mixture for 30 minutes. Add 4- (5-bromo-2-chlorobenzyl) phenol (12.5 g, 42.12 mmol). Stir the mixture for 20 h at room temperature and concentrate. Purify the crude product by column chromatography (PE / EA (v / v) = 10 : 1,) to obtain the product. 1H NMR (400 MHz, CDCl3): δ7.26-7.19 (m, 3 H, ArH), 7.09-7.07 (d, J = 8.5 Hz, 2H, ArH), 6.80-6.78 (d, J = 8.6Hz, 2 H, ArH), 4.89-4.87 (m, 1 H, CH), 4.00-3.94 (m, 5 H, CH, CH2), 3.90-3.86 (m, 1 H, CH), 2.17-2.13 (m, 2 H, CH2) [2].
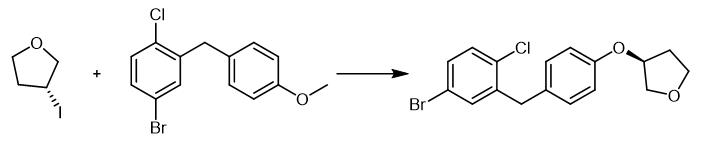
Fig. 4 The synthetic method 3 of (3S)-3-[4-[(5-Bromo-2-chlorophenyl)methyl]phenoxy]tetrahydrofuran.
4-Bromo-1-chloro-2-(4-cyclopentyloxy-benzyl)-benzene : To a mixture of 40.0 g 4-(5-bromo-2-chloro-benzyl)-phenol and 71.0 g cesium carbonate in 300 ml. ethanol are added 23 ml. iodocyclopentane. The mixture is stirred at 60 °C over night and then cooled to ambient temperature. The ethanol is evaporated, and water is added to the residue. The resulting mixture is extracted with ethyl acetate, the combined extracts are dried over sodium sulfate, and the solvent is removed. The residue is filtered through silica gel (cyclohexane/ethyl acetate 100:1-10:1). 4-Bromo-1-chloro-2-(4-cyclopentyloxy-benzyl)-benzene. Yield: 34.4 g (70% of theory). (1 ) (S)-4-bromo-1-chloro-2-(4-tetrahydrofuran-3-yloxy-benzyl)-benzene. Mass spectrum (ESI+): m/z = 366/368/370 (Br+CI) [M+H]+[3].
Application
For the preparation of empagliflozin
Liu et al. provide a new crystal form of (3S)-3-[4-[(5-bromo-2-chlorophenyl)methyl]phenoxy]tetrahydrofuran and a preparation method of empagliflozin, the new crystal The type has a higher melting point and lower hygroscopicity. The new crystal form grows crystal to form a crystal nucleus within a pressure range suitable for industrial production conditions, and is prepared by cooling and water-promoted crystallization. The preparation method has simple reaction, high operability, good product quality and high yield. , is a preparation process suitable for industrialization [4].
Improve concrete strength
Aiming at the problem that the structural strength of permeable concrete decreases, a permeable concrete is provided, and the technical scheme is as follows: the following components are included in parts by mass: 100 parts of Portland cement; 550-600 parts of coarse aggregate; (3S)- 15-30 parts of 3-[4-[(5-bromo-2-chlorophenyl)methyl]phenoxy]tetrahydrofuran; 90-110 parts of water. By adding (3S)-3-[4-[(5-bromo-2-chlorophenyl)methyl]phenoxy]tetrahydrofuran to the permeable concrete, the physical properties of the permeable concrete are effectively improved, and the strength of the pervious concrete is improved , so that it is suitable for more projects with high strength requirements for concrete, with a wide range of applications and wide applicability [5].
References
[1] Wang X, Zhang L, Byrne D, et al. Efficient synthesis of empagliflozin, an inhibitor of SGLT-2, utilizing an AlCl3-promoted silane reduction of a β-glycopyranoside[J]. Organic letters, 2014, 16(16): 4090-4093.
[2] Li Y, Shi Z, Chen L, et al. Discovery of a potent, selective renal sodium-dependent glucose cotransporter 2 (SGLT2) inhibitor (HSK0935) for the treatment of type 2 diabetes[J]. Journal of Medicinal Chemistry, 2017, 60(10): 4173-4184.
[3] Eckhardt M, Himmelsbach F, Wang X-J, et al. Processes for the preparation of glucopyranosyl-substituted benzyl or benzene derivatives[P]. PCT Int. Appl., 2006120208, 2006.
[4] Liu Z, Bai X, Mei G, et al. New crystalline form of (3S)-3-(4-((5-bromo-2-chlorophenyl)methyl)phenoxy)tetrahydrofuran, useful as intermediate for preparing empagliflozin[P]. Faming Zhuanli Shenqing, 113666892, 2021.
[5] Cao W, Fu R, Cao S. Water-permeable concrete comprises Portland cement, aggregate, (3S)-3-(4-((5-bromo-2-chlorophenyl)methyl)phenoxy) tetrahydrofuran and water[P]. Faming Zhuanli Shenqing, 110563408, 2019.
Related articles And Qustion
See also
Lastest Price from (3S)-3-[4-[(5-Bromo-2-chlorophenyl)methyl]phenoxy]tetrahydrofuran manufacturers
![915095-89-5 (3S)-3-[4-[(5-Bromo-2-chlorophenyl)methyl]phenoxy]tetrahydrofuran](/ProductImageEN1/2025-08/Small/08906153-6b3c-4732-ae50-d128d8457f75.jpg)
US $1.00/g2025-08-11
- CAS:
- 915095-89-5
- Min. Order:
- 100g
- Purity:
- 99
- Supply Ability:
- 1000
![915095-89-5 (3S)-3-[4-[(5-Bromo-2-chlorophenyl)methyl]phenoxy]tetrahydro-furan](/ProductImageEN/2021-11/Small/16637782-df5c-40ce-b6a7-515f1ad9d660.jpg)
US $0.00-0.00/Kg/Drum2025-04-21
- CAS:
- 915095-89-5
- Min. Order:
- 1KG
- Purity:
- 99%min
- Supply Ability:
- 1000kg
![915095-89-5 (3S)-3-[4-[(5-Bromo-2-chlorophenyl)methyl]phenoxy]tetrahydrofuran; Synthesis; Application](https://www.chemicalbook.com/CAS/20150408/GIF/915095-89-5.gif)