synthesis
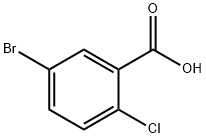
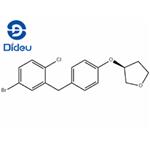
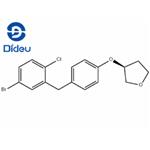
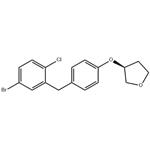
To a mixture of AlCl3 (61.7 g, 0.463 mol) and (S)-(5-bromo-2-chlorophenyl)(4-((tetrahydrofuran-3-yl)oxy)phenyl)methanone (100 g, 0.231 mol) in toluene (450 mL) was added 1,1,3,3-tetramethyldisiloxane (40.4 g, 0.301 mol) over 1 hour at<20 °C. The mixture was held at 20-23 °C for an additional 1.5 hours and cooled to 0-5 °C. The cooled solution was slowly added to ice-water (400 mL) over 15 min and the layers separated. The organic was treated with 3N NaOH (400 mL) at room temperature for 16-24 hours and the layers were separated. The organic was concentrated to a low volume (~130 mL). After diluted with acetonitrile (450 mL), the solution was again concentrated to a low volume (~130 mL). To the residue was added acetonitrile (400 mL) followed by a slow addition of water (200 mL). The resulting mixture was cooled to 0-3 °C and held for 2 hours. The solids were collected by filtration and dried in oven to give product. (S)-3-(4-(2-chloro-5- bromobenzyl)phenoxy)tetrahydrofuran (2a). 92% as white crystals.
![synthesis of (3S)-3-[4-[(5-Bromo-2-chlorophenyl)methyl]phenoxy]tetrahydrofuran synthesis of (3S)-3-[4-[(5-Bromo-2-chlorophenyl)methyl]phenoxy]tetrahydrofuran](https://www.chemicalbook.com/NewsImg/2022-08-16/6379623720565357024820368.jpg)
![(3S)-3-[4-[(5-Bromo-2-chlorophenyl)methyl]phenoxy]tetrahydrofuran Structure](https://www.chemicalbook.com/CAS/20150408/GIF/915095-89-5.gif)

![synthesis of (3S)-3-[4-[(5-Bromo-2-chlorophenyl)methyl]phenoxy]tetrahydrofuran synthesis of (3S)-3-[4-[(5-Bromo-2-chlorophenyl)methyl]phenoxy]tetrahydrofuran](https://www.chemicalbook.com/NewsImg/2022-08-16/6379623720565357024820368.jpg)