Synthesis and Application of 3,5-Dichlorobenzoyl chloride
General description
3,5-Dichlorobenzoyl chloride is an important intermediate of pesticide, medicine and dye. In pesticide production, pesticides can be prepared by benzoic acid reaction; In the field of medicine, headache drugs and antidiuretic hormone drugs can be prepared.
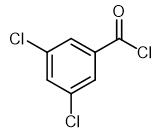
Fig. 1 The structure of 3,5-Dichlorobenzoyl chloride.
Synthetic routes
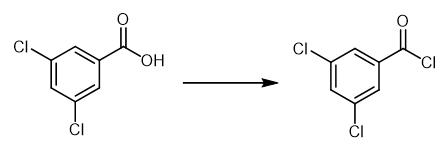
Fig. 2 The synthetic method 1 of 3,5-Dichlorobenzoyl chloride.
Dissolve aryl carboxylic acid (10.0 mmol) in 50 mL DCM with drops of DMF to a 100 mL roundbottomed flask. Cool the mixture to 0°C. Add oxalyl chloride (20.0 mmol, 2.0 equivalents) dropwise to the reaction mixture. Allow the reaction mixture to react for another 4 hours. Concentrate the solvent in vacuo. Use the remaining residue directly [1].
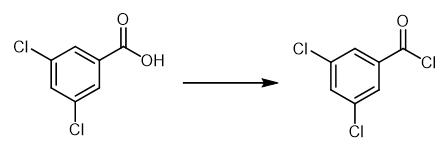
Fig. 3 The synthetic method 2 of 3,5-Dichlorobenzoyl chloride.
Heat a solution of 3,5-dimethylbenzoic acid (300 mg, 2.00 mmol) in thionyl chloride (1.2 mL, 16 mmol) to reflux. Allow the mixture to react for 18 hours and cool the reaction mixture. Remove the thionyl chloride under vacuum with the assistance of excess toluene as an azeotrope to obtain product [2].
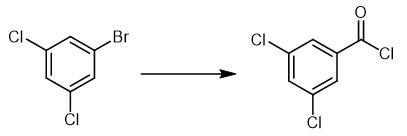
Fig. 4 The synthetic method 3 of 3,5-Dichlorobenzoyl chloride.
Add aryl bromide (1.25 mmol), benzyltriphenyl phosphonium chloride (727 mg, 1.88 mmol, 1.5 equivalents), Pd(PtBu3)2 (32 mg, 0.0625 mmol, 5 mol%) and a stir bar in a steel Parr autoclave in a nitrogen glove box. Place the autoclave in a Parr system. Charge the system with carbon monoxide by evacuating and filling the system three times. Finally pressurize the system to 20 atmosphere CO. Heat the reaction mixture to 110 °C for 24 hours with a stirring rate of 600 RPM. Allow the vessel to cool to room temperature. Evacuate the headspace. Bring back the vessel into the glove box. Filter the crude mixture over a fine sintered glass frit to remove the insoluble phosphonium salt. Rinse the mixture with toluene (3 × 1 mL) [3].
Application
Synthesis of dichlorobenzamide derivatives
Reactions of 3,5-dichlorobenzoyl chloride and arylamine compounds in N,N '-dimethylformamide solution at 60 degrees C afforded a series of dichlorobenzamide derivatives 4-14 in good yields. These new compounds and accompanying intermediates were characterized and confirmed by nuclear magnetic resonance and infrared spectroscopy, of which structures of compounds 4 and 6 were established by X-ray crystallography. Compound 4 crystallizes in triclinic space group Pi, with a = 5.047 (18), b = 10.28 (4), c = 13.36 (5) angstrom, alpha = 107.59 (5)degrees, beta = 93.55 (5)degrees, gamma = 98.34 (5)degrees, and Z = 2. The unit cell of 6 has a monoclinic Pn symmetry with the cell parameters a = 9.581 (3), b = 12.217 (4), c = 11.072 (3) angstrom, beta = 92.584 (4)degrees, and Z = 4 [4].
Synthesis of 5,7-dichlorotetrahydroisoquinoline-6-carboxylic acid
The development of an industrially scalable synthetic route for 5,7-dichlorotetrahydroisoquinoline-6-carboxylic acid (1), a key starting material for lifitegrast, is described. This route includes the following features: (1) tetrahydroisoquinoline 19 was prepared in three steps from readily commercially available 3,5-dichlorobenzoyl chloride and 2-aminoethanol in a high total yield of 76%; (2) formaldehyde instead of triphenylmethyl chloride was employed in the protection of the secondary amine, resulting in much higher atom economy; (3) the target compound 1 was produced in an overall yield of 66% with an HPLC purity of 99% by area [5].
As the ligand backbone
Stereodynamic ligands offer intriguing possibilities in enantioselective catalysis. "NU-BIPHEPs" are a class of stereodynamic diphosphine ligands which are easily accessible via rhodium-catalyzed double [2 + 2 + 2] cycloadditions. This study explores the preparation of differently functionalized "NU-BIPHEP(O)" compounds, the characterization of non-covalent adduct formation and the quantification of enantiomerization barriers. In order to explore the possibilities of functionalization, Storch et al. studied modifications of the ligand backbone, e.g., with 3,5-dichlorobenzoyl chloride. Diastereomeric adducts with Okamoto-type cellulose derivatives and on-column deracemization were realized on the basis of non-covalent interactions. Enantioselective dynamic HPLC (DHPLC) allowed for the determination of rotational barriers of Delta G(298K)(double dagger) = 92.2 +/- 0.3 kJ mol(-1) and 99.5 +/- 0.1 kJ mol(-1) underlining the stereodynamic properties of "NU-BIPHEPs" and "NU-BIPHEP(O)s", respectively. These results make the preparation of tailor-made functionalized stereodynamic ligands possible and give an outline for possible applications in enantioselective catalysis [6].
References
[1] Yang F, Wang X, Zhao W, et al. Hypervalent Iodine (III)-Promoted C3–H Regioselective Halogenation of 4-Quinolones under Mild Conditions[J]. ACS omega, 2021, 6(49): 34044-34055.
[2] Pribut N, Veale C G L, Basson A E, et al. Application of the Huisgen cycloaddition and ‘click’reaction toward various 1, 2, 3-triazoles as HIV non-nucleoside reverse transcriptase inhibitors[J]. Bioorganic & Medicinal Chemistry Letters, 2016, 26(15): 3700-3704.
[3] Quesnel J S, Kayser L V, Fabrikant A, et al. Acid chloride synthesis by the Palladium‐Catalyzed chlorocarbonylation of aryl bromides[J]. Chemistry–A European Journal, 2015, 21(26): 9550-9555.
[4] Zhang J, Li Y, Wang B, et al. Synthesis of Dichlorobenzamide Derivatives: Crystal Structures of 3, 5-Dichloro-N-(2-chlorophenyl) benzamide and 3, 5-Dichloro-N-(4-chlorophenyl) benzamide[J]. Journal of Chemical Crystallography, 2021, 51(1): 108-115.
[5] Xu W, Gong X, Odilov A, et al. Scalable Process for Making 5, 7-Dichlorotetrahydroisoquinoline-6-carboxylic Acid Using Methylene as the Protecting Group[J]. Organic Process Research & Development, 2021, 25(11): 2447-2452.
[6] Storch G, Pallmann S, Rominger F, et al. Stereodynamic tetrahydrobiisoindole “NU-BIPHEP (O)” s: functionalization, rotational barriers and non-covalent interactions[J]. Beilstein journal of organic chemistry, 2016, 12(1): 1453-1458.
You may like
See also
Lastest Price from 3,5-Dichlorobenzoyl chloride manufacturers

US $0.00/kg2025-05-21
- CAS:
- 2905-62-6
- Min. Order:
- 1000kg
- Purity:
- 3,5-DCBC (GC) 95+
- Supply Ability:
- 20000mt

US $100.00-75.00/kg2025-04-21
- CAS:
- 2905-62-6
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 5000Ton