Synthesis and application of 2-(Diethylamino)ethyl methacrylate
2-(Diethylamino)ethyl methacrylate is a colorless to almost colorless liquid under ambient conditions, characterized by high chemical reactivity and distinctive polymerization properties. It exhibits slight solubility in water but is miscible with most organic solvents. Possessing both a reactive amino group and a polymerizable vinyl group, 2-(Diethylamino)ethyl methacrylate can enhance adhesion and thermosetting characteristics, while also improving crosslinking capability as well as dye and pigment coloring performance due to ionic interactions, making it suitable for applications in the preparation of thermosetting coatings, antistatic agents, dyeing auxiliaries, lubricant additives, adhesives, leather processing agents, and combustion improvers.

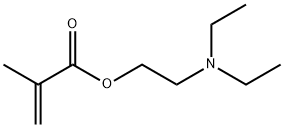
Figure1: Picture of 2-(Diethylamino)ethyl methacrylate
Synthesis
Method 1
Methacryloyl chloride was first synthesized via the oxalyl chloride method, using methacrylic acid and oxalyl chloride as starting materials with a molar ratio of n(methacrylic acid) : n(oxalyl chloride) = 1 : 1.08. Subsequently, 2-(Diethylamino)ethyl methacrylate (DEAM) was prepared through an acylation reaction between diethylaminoethanol and the as-synthesized methacryloyl chloride, using diethyl ether as the solvent under a molar ratio of n(methacryloyl chloride) : n(diethylaminoethanol) = 1 : 1.6. This versatile monomer, 2-(Diethylamino)ethyl methacrylate, was successfully obtained after reacting in an ice bath for 4 hours, achieving a high yield of 94.4% relative to methacryloyl chloride. [1]
Method 2
An improved process for synthesizing 2-(Diethylamino)ethyl methacrylate via transesterification of methyl methacrylate and diethylaminoethanol, using lithium hydroxide as a catalyst, was systematically investigated. In this process, n-hexane served as a methanol-entraining solvent, with a water separator being employed to continuously remove methanol from the reaction system. The optimized reaction conditions were determined as follows: n(methyl methacrylate) : n(diethylaminoethanol) : n(catalyst) = 2.4 : 1 : 0.42, a reaction time of 6 hours, and a temperature of approximately 70°C. Under these specified conditions, the content of the target product, 2-(Diethylamino)ethyl methacrylate, reached 94.96% with an isolated yield of 93.16%. This improved methodology offers significant advantages, including mild reaction conditions, the use of a low-cost and readily available catalyst, high product content and yield, as well as operational simplicity. [2]
Application
Poly(2-(diethylamino)ethyl methacrylate) (PDEAEMA) and its quaternized derivatives have garnered significant attention as versatile water-soluble polymers, particularly for applications in environmental protection, while the temperature-sensitive solubility profile of PDEAEMA further extends its potential utility to advanced domains such as drug delivery systems and sensors. As a building block for amphiphilic block copolymers, 2-(diethylamino)ethyl methacrylate enables the formation of micellar structures that serve as effective stabilizers in dispersion polymerization processes. Although controlled polymerization of 2-(diethylamino)ethyl methacrylate has been achieved through living anionic polymerization and group transfer polymerization, both methods present limitations: the former demands rigorously controlled reaction conditions that hinder industrial-scale implementation, while the latter restricts copolymer formation to other (meth)acrylate comonomers. More recently, stable free radical polymerization has been employed to synthesize polystyrene-PDEAEMA block copolymers; however, this approach failed to provide control over the molecular weight of the PDEAEMA block and was unsuccessful in achieving homopolymerization of the monomer. [3]
Preparation of polyampholyte microgels
pH-responsive polyampholyte microgels, designated as P(MAA-co-DEA), were synthesized via inverse microemulsion polymerization using methacrylic acid (MAA) and 2-(Diethylamino)ethyl methacrylate (DEA) as comonomers, N,N'-methylenebisacrylamide (MBA) as crosslinker, and poly(ethylene glycol) methacrylate (PEGMA) as macromolecular stabilizer. The morphology and swelling properties of the resulting microgels were characterized by transmission electron microscopy and dynamic light scattering. Using bovine serum albumin (BSA) as a model drug, the effects of crosslinker content, pH, and ionic strength on the release behavior were investigated by UV spectrophotometry, and the underlying release mechanism was preliminarily studied. The results indicated that the hydrodynamic diameter and swelling ratio of the microgels at both low and high pH were greater than those at the isoelectric point (IEP); the release of BSA was dependent on the crosslinker content, with an optimal value observed. [4]
Reference
[1] He L Z, Zeng Q Y, Xu R A. Synthesis of diethylaminoethyl methacrylate[J]. Fine Chemical Intermediates, 2009, 39(6): 37-38.
[2] Zhu F L, Sui X T, Zhao J, et al. Improvement of synthesis process for diethylaminoethyl methacrylate[J]. Journal of Qingdao University of Science and Technology: Natural Science Edition, 2018, 39: 30-33.
[3] Zhang X, Xia J, Matyjaszewski K. Controlled/“living” radical polymerization of 2-(dimethylamino) ethyl methacrylate[J]. Macromolecules, 1998, 31: 5167-5169.
[4] Ren Y N, Shen Y H, Zhang W X, et al. Properties and release behavior of polyampholyte microgel P(MAA-co-DEA)[J]. Journal of Taiyuan University of Technology, 2013, 44: 491-495.
You may like
Lastest Price from 2-(Diethylamino)ethyl methacrylate manufacturers
US $10.00/KG2025-04-21
- CAS:
- 105-16-8
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $0.00/KG2025-04-15
- CAS:
- 105-16-8
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 500000kg