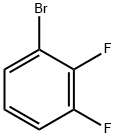
Synthesis and Application of 2,3-Difluorobromobenzene
6H3BrF2, and the CAS is 38573-88-5. 2,3-Difluorobromobenzene is a colorless to pale yellow liquid with a boiling point of 234 °C. At 25°C, its density is 1.724 g/mL.

Synthetic routes
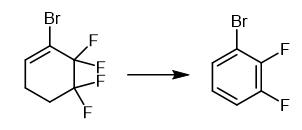
Fig. 2 The synthetic method 1 of 1-Bromo-2,3-difluorobenzene.
Add 50% aqueous solution of KOH (18.0 g, 160 mmol) to a mixture of 1-bromo-5,5,6,6-tetrafluorocyclohex-1-ene (11.65 g) and triethylbenzylammonium chloride (0.15 g, 0.7 mmol) at 30-35°C for 30 minutes. Keep the reaction mixture at 75-85°C for 2 hours. Cool the reaction mixture. Dilute the reaction mixture with water. Extract the organic product with CH2Cl2. Dry the organic product over CaCl2. Distill the organic product. 1H NMR (CDCl3, 300.1 MHz), δ: 7.00-7.16 (m, 1H, Ar); 7.17-7.29 (m, 1H, Ar); 7.34-7.47 (m, 1H, Ar). 13C NMR (CDCl3, 75.5 MHz), δ: 110.40 (d, C(1), J = 17.5 Hz); 116.40 (d, C(4), J = 17.7 Hz); 124.70 (dd, C(5), J = 7.1 Hz, J = 5.0 Hz); 128.23 (d, C(6), J = 3.6 Hz); 148.10 (dd, C(2), J = 248.8 Hz, J = 14.3 Hz); 150.92 (dd, C(3), J = 251.9 Hz, J = 13.3 Hz). 19F NMR (CDCl3, 282.4 MHz), δ: -130.9 (m, 1 F, Ar), -134.8 (m, 1 F, Ar). BP 157-158°C. Elemental Analysis Found (%): C, 37.54; H, 1.50. C6H3BrF2. Calculated (%): C, 37.34; H, 1.52. Mass Spec MS, m/z (Irel (%)): 194, 192 [M]+ (100, 99), 113 [M-Br]+ (88), 63 (60) [1].
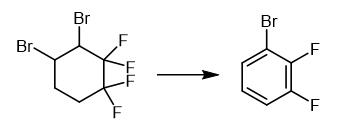
Fig. 3 The synthetic method 2 of 1-Bromo-2,3-difluorobenzene.
Add a 50% aqueous solution of KOH (140.0 g, 1.25 mol) to a mixture of 3,4-dibromo-1,1,2,2-tetrafluorocyclohexane (100.0 g) and triethylbenzylammonium chloride (0.94 g, 4 mmol) maintaining temperature in a range of 20-30°C for 1.5 hours. Stir the reaction mixture at 80-85°C for 2 hours. Distill off the organic product with water vapor. Dry the organic product over CaCl2. Distill the organic product. 1H NMR (CDCl3, 300.1 MHz), δ: 7.00-7.16 (m, 1H, Ar); 7.17-7.29 (m, 1H, Ar); 7.34-7.47 (m, 1H, Ar). 13C NMR (CDCl3, 75.5 MHz), δ: 110.40 (d, C(1), J = 17.5 Hz); 116.40 (d, C(4), J = 17.7 Hz); 124.70 (dd, C(5), J = 7.1 Hz, J = 5.0 Hz); 128.23 (d, C(6), J = 3.6 Hz); 148.10 (dd, C(2), J = 248.8 Hz, J = 14.3 Hz); 150.92 (dd, C(3), J = 251.9 Hz, J = 13.3 Hz). 19F NMR (CDCl3, 282.4 MHz), δ: -130.9 (m, 1 F, Ar), -134.8 (m, 1 F, Ar). Mass Spec MS, m/z (Irel (%)): 194, 192 [M]+ (100, 99), 113 [M-Br]+ (88), 63 (60) [1].
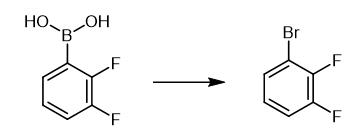
Fig. 4 The synthetic method 3 of 1-Bromo-2,3-difluorobenzene.
A-bromosuccinimide (78.30 g, 0.440 mol) was added to a stirred suspension of compound 5 (35.00 g, 0.222 mol) in acetonitrile (300 ml) under dry nitrogen. The stirred mixture was heated under reflux for 16 h (GLC and TLC analysis revealed a complete reaction) and poured into water. The product was extracted into hexane (×2), and the com bined organic extracts were washed successively with sodium bisulphite and sodium bicarbonate and dried (MgSO4). The solvent was distilled off at atmosphere pressure (68°C) and the product was distilled at atmospheric pressure. Yield 30.0 g (70%). bp 158°C. 1H NMR (400 MHz, CDCl3), δ 6.98(1H, dddd), 7.11(1H, dddd), 7.29(1H, dddd). MS m/z 194(M+), 192(M+) [2].
Application
New metabolites from the microbial oxidation of fluorinated aromatic compounds
m-Bromo-alpha,alpha,alpha-trifluorotoluene (1) and 1-bromo-2,3-difluorobenzene (4) were subjected to microbial oxidation by Pseudomonas putida strain 39/D and the corresponding Escherichia coli recombinant microorganism (Strain JM109(pDTG601)), which express toluene dioxygenase. The absolute stereochemistry of the major oxidation products have been determined as cis-(2R,3S)-5-bromo-2,3-dihydroxy-alpha,alpha,alpha-trifluoromethylcyclohexa-4,6-diene (2), and cis-(2S,3S)-1-bromo-5,6-difluoro-2,3-dihydroxy-4,6-diene (5). The regiochemistry of a minor metabolite has been established as cis-5-bromo-3,4-dihydroxy-alpha,alpha,alpha-trifluoromethylcyclohexa-1,5-diene (3) [3].
Regioselective synthesis of 2,3-difluorohalobenzenes
The gas-phase copyrolysis of tetrafluoroethylene and buta-1,3-diene in a flow tube reactor at 490-510 degrees C gives 3,3,4,4-tetrafluorocyclohex-1-ene, which is selectively converted to 1-bromo- or 1-chloro-2,3-difluorobenzene via intermediate steps of halogenation and dehydrohalogenation [4].
References
[1] Volchkov N V, Lipkind M B, Nefedov O M, et al. Three-step regioselective synthesis of 2, 3-difluorohalobenzenes using tetrafluoroethylene and buta-1, 3-diene as starting building blocks[J]. Russian Chemical Bulletin, 2021, 70(5): 925-932.
[2] Radini I A, Hird M. The synthesis and mesomorphic properties of liquid crystals with bulky terminal groups designed for bookshelf geometry ferroelectric mixtures[J]. Liquid Crystals, 2009, 36(12): 1417-1430.
[3] Hudlicky T, Gonzalez D, Stabile M, et al. New metabolites from the microbial oxidation of fluorinated aromatic compounds[J]. Journal of fluorine chemistry, 1998, 89(1): 23-30.
[4] Volchkov N V, Lipkind M B, Nefedov O M, et al. Three-step regioselective synthesis of 2, 3-difluorohalobenzenes using tetrafluoroethylene and buta-1, 3-diene as starting building blocks[J]. Russian Chemical Bulletin, 2021, 70(5): 925-932.
See also
Lastest Price from 1-Bromo-2,3-difluorobenzene manufacturers

US $0.00-0.00/mg2025-06-05
- CAS:
- 38573-88-5
- Min. Order:
- 10mg
- Purity:
- 99%+ HPLC
- Supply Ability:
- 1000
US $0.00/kg2025-03-03
- CAS:
- 38573-88-5
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10000KGS