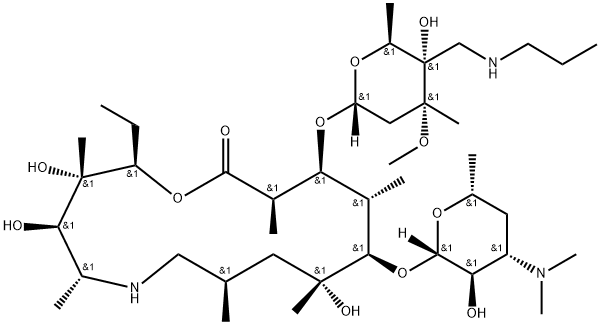
Function and pharmacokinetic parameters of tulathromycin A
Introduction
Tulathromycin A is a new macrolide antibiotic specially used for mammals, and it is a drug developed by Pfizer Animal Health Company in the United States for respiratory diseases in cattle and pigs[1].
Picture 1 Tulathromycin A tablets
Function and pharmacokinetic parameters
Tulathromycin A is a broad-spectrum antibiotic. After administration, the drug is rapidly absorbed, has high bioavailability, long half-life, lasting efficacy, can be widely distributed in body tissues, has good cell permeability, and the drug concentration in lung tissue is higher than that in blood. The single dose of 2.5 mg/kg body weight of this product is absorbed rapidly and completely after subcutaneous injection. After 15 minutes, the blood concentration reaches the peak, and the drug concentration in the lung remains high and lasting. The relative bioavailability exceeds 90%, the clearance rate (Cl) is 170 mL/hr/kg, and the apparent distribution volume (Vd) was 11 L/kg, 2.75 days in plasma and 8.75 days in lung. 15 minutes after intramuscular or intravenous injection of 2.5 mg/kg in pigs: the plasma concentration reached a peak of 616ng/mL, and the average elimination half-life of plasma was 75.6h; After intravenous injection, the plasma clearance rate (Clp) was 181 mL/kg/h, the steady-state apparent distribution volume (Vss) was 13.2 L/kg, and the elimination half-life was 67.5 h. The bioavailability of intramuscular injection was more than 87%. AUCinf in lung tissue was 61.4 times of AUCinf in plasma, and the elimination half-life of lung was 142 h (6 days). This shows that the drug was rapidly absorbed and the bioavailability was high after a single dose of 2.5 mg/kg of tulathromycin A was injected intramuscularly in pigs, The drug concentration in lung tissue is always high, and the elimination is slow.
Efficacy verification
Tulathromycin A has broad-spectrum bacteriostasis and bactericidal effect by inhibiting bacterial protein synthesis by blocking bacterial peptide transfer process, and is sensitive to some gram-positive and gram-negative bacteria, especially to pathogenic bacteria of respiratory diseases of cattle and pigs, such as Actinobacillus pleuropneumoniae, Pasteurella haemolytica, Pasteurella haemosepticus, Mycoplasma pneumoniae, Haemophilus parasuis, Bordetella bronchisepticus, etc. According to the reports of Hart FJ and Nutsch RG, the product is injected intramuscularly with tulathromycin A at a single dose of 2.5 mg/kg
In the treatment of actinobacillus pleuropneumonia artificially infected pigs, there was no significant difference between the effect of repeated administration of ceftiofur for 3 consecutive days. McKelvie J and others reported; The effect of single intramuscular injection of 2.5 mg/kg of this product in the treatment of mycoplasma pneumonia in artificially infected pigs is better than that of anthracofloxacin for 5 days; Nanjiani IA et al. reported that 349 pigs naturally infected with respiratory diseases were treated with a single intramuscular injection of 2.5 mg/kg of this product, and the effect was equivalent to that of continuous application of florfenicol for 2 days; Rooney KA uses this product to prevent respiratory diseases in high-risk cattle by subcutaneous injection, which is more effective than tulathromycin A and florfenicol. Because of its good pharmacokinetics and excellent in vitro antibacterial activity against pathogenic bacteria of respiratory diseases, tulathromycin A has become an effective drug that can prevent and treat respiratory diseases of pigs and cattle with a single dose of injection.
Adverse effects and precautions
Tulathromycin A has no carcinogenicity, teratogenicity and genotoxicity, and will not induce gene mutation; However, it may easily cause cardiac toxicity. After administration, cattle and pigs will have short-term (generally less than 4 hours) hypersalivation or dyspnea, and there will be local reactions after injection. Therefore, the use of tulathromycin A for the prevention and treatment of respiratory diseases in cattle and pigs must be conducted under the guidance of veterinarians, and cannot be used for lactating cows and non ruminant calves; The drug withdrawal period of cattle is 18 days; The drug withdrawal period of pigs is 5 days; When the drug is injected under the cow skin, each injection site should not exceed 10ml; When pigs are injected intramuscularly, each injection site should not exceed 2.5 ml. Therefore, The prevention and treatment of respiratory diseases of cattle and pigs with bacteriocin must be carried out under the guidance of veterinarians.
Reference
1 Tolamycin, a new macrolide antibiotic for animals [J]. Northern Animal Husbandry, 2012 (07): 29
Related articles And Qustion
See also
Lastest Price from Tulathromycin A manufacturers
US $0.00/kg2025-11-19
- CAS:
- 217500-96-4
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- Customise

US $0.00-0.00/KG2025-04-21
- CAS:
- 217500-96-4
- Min. Order:
- 10g
- Purity:
- 96%-102%
- Supply Ability:
- 50KG