Uses
1-Bromo-2,3-difluorobenzene may be used in the synthesis of potent, orally active Calcitonin gene-related peptide (CGRP) receptor antagonist (BMS-846372). It may be used in the preparation of 2,3-difluorophenyl(dimethyl)phosphane.
Synthesis
Add
50% aqueous solution of KOH (18.0 g, 160 mmol) to a mixture of
1-bromo-5,5,6,6-tetrafluorocyclohex-1-ene (11.65 g) and
triethylbenzylammonium chloride (0.15 g, 0.7 mmol) at 30-35°C for 30
minutes. Keep the reaction mixture at 75-85°C for 2 hours. Cool the
reaction mixture. Dilute the reaction mixture with water. Extract the
organic product with CH2Cl2. Dry the organic product over CaCl2. Distill the organic product. 1H NMR (CDCl3, 300.1 MHz), δ: 7.00-7.16 (m, 1H, Ar); 7.17-7.29 (m, 1H, Ar); 7.34-7.47 (m, 1H, Ar). 13C NMR (CDCl3,
75.5 MHz), δ: 110.40 (d, C(1), J = 17.5 Hz); 116.40 (d, C(4), J = 17.7
Hz); 124.70 (dd, C(5), J = 7.1 Hz, J = 5.0 Hz); 128.23 (d, C(6), J = 3.6
Hz); 148.10 (dd, C(2), J = 248.8 Hz, J = 14.3 Hz); 150.92 (dd, C(3), J =
251.9 Hz, J = 13.3 Hz). 19F NMR (CDCl3, 282.4 MHz), δ: -130.9 (m, 1 F, Ar), -134.8 (m, 1 F, Ar). BP 157-158°C. Elemental Analysis Found (%): C, 37.54; H, 1.50. C6H3BrF2. Calculated (%): C, 37.34; H, 1.52. Mass Spec MS, m/z (Irel (%)): 194, 192 [M]+ (100, 99), 113 [M-Br]+ (88), 63 (60).
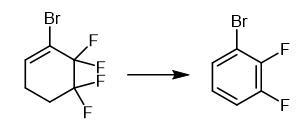
Fig The synthetic method of 1-Bromo-2,3-difluorobenzene

 Fig The synthetic method of 1-Bromo-2,3-difluorobenzene
Fig The synthetic method of 1-Bromo-2,3-difluorobenzene