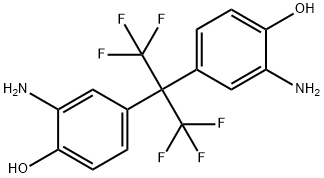
Synthesis and Application of 2,2-bis(3-amino-4-hydroxyphenyl)hexafluoropropane
General description
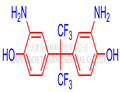
2,2-Bis(3-amino-4-hydroxyphenyl)hexafluoropropane (6FAP) is white crystal. This product is non-toxic and can be stored at room temperature away from light and air.
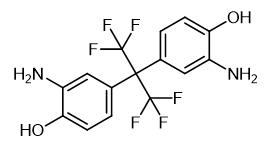
Fig. 1 The structure of 2,2-Bis(3-amino-4-hydroxyphenyl)hexafluoropropane.
Physicochemical property
2,2-Bis(3-amino-4-hydroxyphenyl)hexafluoropropane is a white solid with a melting point of 245-248 °C. Its boiling point and density are expected to be 411.3±45.0 °C and 1.545±0.06 g/cm3, respectively. It is slightly soluble in DMSO and methanol.
Synthetic routes
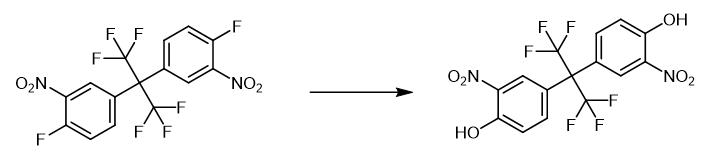
Fig. 2 The synthetic step 1 of 2,2-Bis(3-amino-4-hydroxyphenyl)hexafluoropropane.
50 g bis(3-nitro-4-fluorophenyl) hexafluoropropane is dropped into the 500 mL four-necked flask, 400 g DMSO is added, the stirring is turned on and be warming up to 70° C. 30 g potassium hydroxide powder is added, and the temperature of the kettle is increased to 80 °C. React at -85°C, stop the reaction when the content of bis(3-nitro-4-chlorophenyl)hexafluoropropane is less than 1.0%, the target product bis(3-nitro-4-hydroxyphenyl)hexafluoropropane The gas phase content is 98%, and the system is black, and the still temperature is reduced to room temperature, and 500 mL of water is added, and 50 g of 30% hydrochloric acid is added dropwise and neutralized to pH=2, and filtered to obtain two (3-nitro-4-hydroxyphenyl) six Crude fluoropropane; recrystallize the crude product of compound (IV), the recrystallization solvent is ethanol, and the amount of ethanol is 3 times that of bis(3-nitro-4-hydroxyphenyl)hexafluoropropane. Base-4-hydroxyphenyl) hexafluoropropane crude product is mixed with ethanol and raises the still temperature to 60 ℃ of stirring and dissolving, then the still temperature is reduced to 10-20 ℃ of crystallization, filters, and oven dry obtains compound bis(3- nitro-4-hydroxyphenyl)hexafluoropropane pure product 45.1 g, the yield is 91.1%, and the purity is 98.6%. 1H-NMR (400 MHz, D6-DMSO) δ (ppm): 7.15(d,2H),7.60(m,2H),7.94(d,2H),14.40(s,2H) [1].
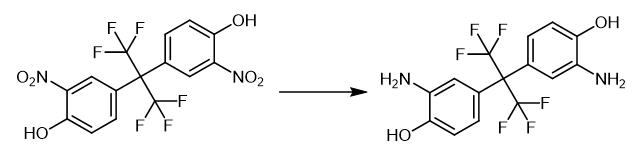
Fig. 3 The synthetic step 2 of 2,2-Bis(3-amino-4-hydroxyphenyl)hexafluoropropane.
30 g two (3-nitro-4-hydroxyphenyl) hexafluoropropanes are dropped into the 1000 mL autoclave, add 300 g DMF and 1.5 g palladium/carbon (the mass fraction of palladium in the palladium/carbon is 5%), press hydrogen to 1MPa, open stirring and be warmed up to 70 ℃, stop reaction after two (3-nitro-4-hydroxyphenyl) hexafluoropropane content<0.1%, target product two (3-amino-4-hydroxyphenyl) hexafluoropropane Fluoropropane gas phase content is 98%, and the system is black, and the temperature of the still is lowered to room temperature, filtered, and the filtrate is de-dried under reduced pressure at 70-80°C to obtain bis(3-amino-4-hydroxyphenyl) hexafluoropropane crude product , the crude product is recrystallized, the recrystallization solvent is water and DMF, and the mass ratio of bis(3-amino-4-hydroxyphenyl)hexafluoropropane, water and DMF is 1:4:2. First, the bis(3-nitro-4-hydroxyphenyl) hexafluoropropane crude product is mixed with the recrystallization solvent to increase the still temperature to 60 °C and stir, and then the still temperature is reduced to 10-20 °C for crystallization, filtered, After drying, 25.4 g of pure bis(3-nitro-4-hydroxyphenyl)hexafluoropropane was obtained. The yield of bis(3-amino-4-hydroxyphenyl)hexafluoropropane was 98.3% and the purity was 99.6%. 1H-NMR (400MHz, D6-DMSO) δ (ppm): 4.64 (s, 4H), 6.42 (d, 2H), 7.57 (s, 2H), 6.65 (d, 2H), 9.36 (s, 2H). 19F-NMR (400MHz, D6-DMSO) δ (ppm): -62.78. [1].
Application
Tensile properties
Radiation effects on polyimide (PI) films were studied with different irradiation doses by Co-60. The PI film were synthesized from 2,2-bis(3-amino-4-hydroxyphenyl)hexafluoropropane (Bis-AP-AF) with 3,3',4,4'-Benzophenone tetracarboxylic dianhydride (BTDA). The tensile strength, tensile modulus and elongation at break of the irradiation PI films were measured and compared in order to determine the effect of the irradiation doses on the tensile properties. The results showed that the tensile strength, tensile modulus and elongation at break of the irradiation PI film can be significantly improved by irradiation of Co-60 source. The optimum irradiation doses were 50 kGy. The maximum increment of tensile strength, tensile modulus and elongation at break of the irradiation PI film is 37.8%, 45.0% and 95.2%, respectively [2].
Synthesis of Hydroxyl Group Polyimide
The feasibility of using two step procedures with thermal imidization of polyamic acid (PAA) for synthesizing hydroxyl group polyimide (PI), based on 2,2-Bis(3-amino-4-hydroxyphenyl)hexafluoropropane (Bis-AP-AF), was studied. It was found that a high molecular weight PAA could be successfully synthesized from Bis-AP-AF with 3,3',4,4'-Benzophenone tetracarboxylic dianhydride (BTDA). The optimum polycondensation reaction time on synthesizing molecular weight of the PAA was 4 hours. The PI films could be successfully prepared by thermal imidization of the PAA. The higher the inherent viscosity of the PAA is, the more beneficial for the tensile properties of the PI films. All the results indicate that two step procedures with thermal imidization of the PAA under appropriate conditions represented a promising way for preparation PI films, with a reasonable level of mechanical properties [3].
Synthesis and Properties of a High-Molecular-Weight Polyimide
A soluble polyimide with high molecular weight was synthesized from 4, 4'-(hexafluoroiso propylidene) diphthalic anhydride (6FDA) and 2, 2'-bis(3-amino-4-hydroxyphenyl) hexafluoropropane (BAPAF) via two-step polycondesation procedure involving the preparation of poly(amic acid) (PAA) followed by chemical imidization. Effect of synthesis parameters on polyimide molecular weight involving material ratio, imidization temperature and imidization catalyst were studied. Synthesized PI was analyzed with respect to their molecular weights, chemical structures and solubility through GPC, FTIR, XRD and solubility tests respectively. The results showed that a high-molecular-weight PI was successfully synthesized from 4, 4'-(hexafluoroisopropylidene) diphthalic anhydride and 2, 2'-bis (3-amino-4-hydroxyphenyl) hexafluoropropane with imidization at 80 C for 3h, material ratio of n(BAPAF):n(6FDA) equal to 1:1 and catalyst of n(triethylamine):n (pyridine) less than 1:3. The obtained PI showed excellent solubility in polar aprotic organic solvents such as NMP, DMAc, DMSO, THF and Acetone. Poly(6FDA-BAPAF) PI, with high molecular weight and excellent solubility, which was synthesized under lower imidization temperature that was equal to 80 degrees C, could be easily obtained and convenient to process, thus it is a potential material for membrane separation.
References
[1] Jiang J, Wang C, Cai J. Preparation method of 2,2-bis(3-amino-4-hydroxyphenyl)hexafluoropropane from halobenzene and hexafluoroacetone[P]. Faming Zhuanli Shenqing, 111302944, 2020.
[2] Zheng YH, Zhai Y, Guo JM, et al. Effects of Co-60-irradiation on tensile properties of polyimide based on 2,2-Bis(3-amino-4-hydroxyphenyl
Related articles And Qustion
See also
Lastest Price from 2,2-Bis(3-amino-4-hydroxyphenyl)hexafluoropropane manufacturers
US $0.00-0.00/kg2025-10-27
- CAS:
- 83558-87-6
- Min. Order:
- 1kg
- Purity:
- ≥99.9%
- Supply Ability:
- 50MT

US $25.00/ASSAYS2025-06-27
- CAS:
- 83558-87-6
- Min. Order:
- 100ASSAYS
- Purity:
- 99.5%
- Supply Ability:
- 100 mt