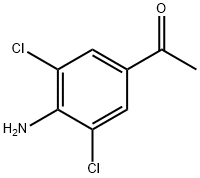
Synthesis and application of 3,5-dichloro-4-aminoacetophenone
General description
3, 5-dichloro-4-aminoacetophenone is beige crystalline and can be used as a pharmaceutical intermediate. CAS number is 37148-48-4, the molecular formula is C8H7Cl2NO and the molecular weight is 204.05. In addition, the melting point is 162-166 °C(lit.), the boiling point is 351.5±42.0°C(Predicted), the density is 1.2748(roughestimate), and the refractive index is 1.5500(estimate). And the Predicted pKa is 1.72±0.10. 3, 5-dichloro-4-aminoacetophenone is slightly dissolved in DMSO and methanol, insoluble in cold water, slightly soluble in hot water, easily soluble in ethanol, ether, benzene and other organic solvents. 3, 5-dichloro-4-aminoacetophenone is an intermediate in the synthesis of the antitussive and antiasthmatic drug gramtin[1-2]. The preparation method is briefly described as follows. Aminoacetophenone and acetic acid (80%) are added into the reaction pot, and are stirred to dissolve them, then quickly adding chlorine-containing glacial acetic acid solution at 5℃ and immediately putting into ice water to precipitate. Finally the crude product is recrystallized with ethanol to get the finished product[3].
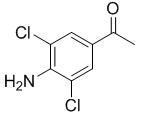
Fig. 1 The structure of 3,5-dichloro-4-aminoacetophenone.
Synthesis
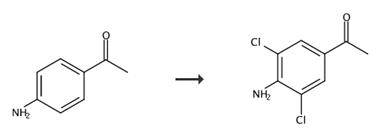
Fig. 2 The synthetic route of 3,5-dichloro-4-aminoacetophenone[4].
Synthesis of α-bromo-4-amino-3,5-dichloroacetophenone. 1.1 Preparation of 4-amino-3,5-dichloroacetophenone (step 1). In a 1-liter 3-necked flask with KPG stirrer and dropping funnel, 11 g (81.4 mmol) of 4-aminoacetophenone is dissolved in 140 ml of glacial acetic acid, the solution is cooled to 15 °C, 140 ml of glacial acetic acid is added into a 250 ml 2-neck flask with gas inlet tube. Chlorine gas is introduced as long as 11 g of chlorine to be dissolved in acetic acid. The chlorine solution is added into the dropping funnel and added rapidly and under vigorous stirring and cooling to the aminoacetophenone solution, immediately after the addition, the mixture is rapidly hydrolyzed with 0.55 liters of ice water, the white precipitate is filtered and recrystallized from ethanol. Yield: 8.69 g (52%). NMR(CDCl3, 500MHz): 2.50 (-CH3, 3H, s); 4.93 (H2N-, 2N, s): 7.82 (arom, H, 2H, s).
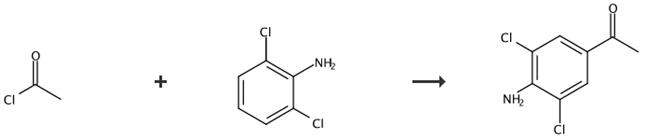
Fig. 3 The synthetic route of 3,5-dichloro-4-aminoacetophenone[5].
Add solid 4'-Aminoacetophenone (approximately 0.6-1.0 mmol) and appropriate molar equivalent of trichloroisocyanuric acid into a 5 mL stainless steel milling jar containing stainless steel balls of diameter 3.0 mm. Grind the admixture subsequently at an appropriate frequency for 0.5 hours at room temperature. After completion of the reaction, monitor the reaction by TLC analyses (petroleum ether/EtOAc) at different time intervals. Take the solid residue into either petroleum ether or a mixture of ethyl acetate/petroleum ether (30%). The both unreacted trihaloisocyanuric acid and cyanuric acid precipitated as insoluble powders. Filter the insoluble material. Evaporate the organic extract.
Application
With the wide application of photosensitive resins in electronic industry, printing industry, coatings, adhesives and medical materials, the research of photoinitiators for photopolymerized photosensitive resins has attracted great attention. In recent years, many efficient, high-quality and practical photoinitiators have been successfully developed for various needs[6-7]. 3,5-dichloro-4-aminoacetophenone is just one of the important ones. Additionally, 3,5-dichloro-4-aminoacetophenon is used to make soap and cigarette[8]. It is also used as an intermediate in organic chemical synthesis[9].
Storage
3,5-dichloro-4-aminoacetophenone should be stored in a cool, dry place in a small, well filled, well-closed container, protected from light. When a partially filled container is used, the air should be replaced by nitrogen or another inert gas. 3,5-dichloro-4-aminoacetophenone oxidizes on exposure to air, resulting in an increase in the peroxide value. It remains clear at 5°C, but darkens in color on standing[10]. Antioxidants are frequently used to extend the shelf life of 3,5-dichloro-4-aminoacetophenone. Protection from oxidation for over 2 years has been achieved by storage in amber glass bottles with the addition of combinations of propyl gallate, butylated hydroxyanisole, butylated hydroxytoluene, and citric or ascorbic acid. A concentration of 0.03% w/v of a mixture of propyl gallate (37.5%), butylated hydroxytoluene (37.5%), and butylated hydroxyanisole (25%) was found to be the best antioxidant for ethyl oleate.
Reference
[1] M. Bantzi, F. Augsburger, J. Loup, Y. Berset, S. Vasilakaki, V. Myrianthopoulos, E. Mikros, C. Szabo, C.G. Bochet, Novel Aryl-Substituted Pyrimidones as Inhibitors of 3-Mercaptopyruvate Sulfurtransferase with Antiproliferative Efficacy in Colon Cancer, J. Med. Chem. 64(9) (2021) 6221-6240.
[2] I. Erol, M.O. Hossoz, Z. Gurler, Synthesis and characterization of new methacrylate copolymers having pendant chloroacetophenon: monomer reactivity ratio, thermal degradation kinetics and biological activity, Polym. Bull. (Heidelberg, Ger.) (2021) Ahead of Print.
[3] C. Fan, H. Lu, Preparation of Clenbuterol hydrochloride, Suzhou Homesun Pharmaceutical Co., Ltd., Peop. Rep. China . 2020, p. 9pp.
[4] M.A. Glushkova, S.V. Popkov, A.M. Martsynkevich, Synthesis and Pharmacokinetics of 2-(4-amino-3,5-dichlorophenyl)-2-(alkylamino)ethanols - Structural Isomers of β2 Agonists Clenproperol and Clenpenterol, Pharm. Chem. J. 54(7) (2020) 694-699.
[5] M.A. Glushkova, S.V. Popkov, A.M. Martsynkevich, M.L. Burdeinyi, Synthesis of β2-Agonist Metabolites of 2-(4-Amino-3,5-Dichlorophenyl)-2-(Alkylamino)Ethanols and their Excretion with Urine in Comparison to the Initial Compounds, Pharm. Chem. J. 55(2) (2021) 142-148.
[6] K.S. Madden, P.M.T. Todd, K. Urata, A.J. Russell, K.A. Vincent, H.A. Reeve, A pharmacophore-based approach to demonstrating the scope of alcohol dehydrogenases, ChemRxiv (2020) 1-64.
[7] K. Murugesan, V.G. Chandrashekhar, T. Senthamarai, R.V. Jagadeesh, M. Beller, Reductive amination using cobalt-based nanoparticles for synthesis of amines, Nat. Protoc. 15(4) (2020) 1313-1337.
[8] K. Murugesan, Z. Wei, V.G. Chandrashekhar, H. Neumann, A. Spannenberg, H. Jiao, M. Beller, R.V. Jagadeesh, Homogeneous cobalt-catalyzed reductive amination for synthesis of functionalized primary amines, Nat. Commun. 10(1) (2019) 1-9.
[9] Y. Wang, H.-y. Liu, W.-l. Chen, Synthesis of five kinds of phenyl ethanolamine β agonists, Huaxue Shiji 40(8) (2018) 805-810.
[10] L. Zhang, W. Cao, S. Han, Preparation method of stable isotope-labeled clenproperol as β2-receptor agonist, Alta Tianjin Standard Material Research Institute Co., Ltd., Peop. Rep. China; Tianjin Alta Scientific Co., Ltd. . 2021, p. 10pp.
Lastest Price from 4-Amino-3,5-dichloroacetophenone manufacturers

US $10.00-1.00/kg2025-10-31
- CAS:
- 37148-48-4
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 300tons

US $180.00/KG2025-09-14
- CAS:
- 37148-48-4
- Min. Order:
- 10000KG
- Purity:
- 99.99%
- Supply Ability:
- 30 TONS