Sulindac sulfide: mechanism of action, activities and safety
General Description
Sulindac sulfide, a nonsteroidal anti-inflammatory drug (NSAID), demonstrates both anti-tumorigenic and anti-inflammatory properties but can cause gastric mucosal damage. Research indicates that it induces autophagic cell death in human and rat gastric epithelial cells by down-regulating Survivin, an apoptosis inhibitor. Additionally, Sulindac sulfide shows promise in treating triple-negative breast cancer by inhibiting Notch1 cleavage and in colon cancer by downregulating Sp transcription factors and related pro-oncogenic gene products. However, safety concerns include potential harm upon ingestion, skin contact, inhalation, and suspected effects on fertility and unborn children, necessitating cautious handling. These findings highlight Sulindac sulfide's potential for alternative NSAID development while emphasizing the importance of safety measures.

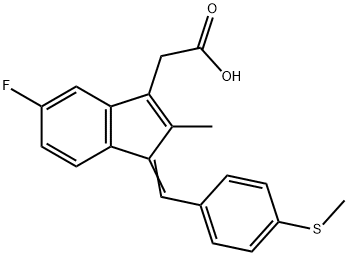
Figure 1. Sulindac sulfide
Mechanism of action
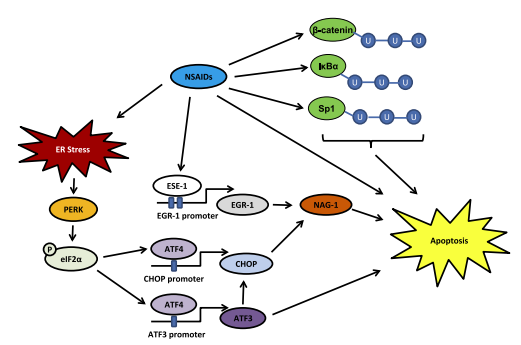
Sulindac sulfide is a nonsteroidal anti-inflammatory drug with both anti-tumorigenic and anti-inflammatory properties. However, it has been shown to cause damage to the gastric mucosa. Studies have found that NSAIDs can cause gastric injury by down-regulating Survivin, an apoptosis inhibitor, leading to apoptosis induction. Autophagy is a process that promotes cellular health by destroying unwanted cellular materials, but excessive autophagy induction could lead to a non-apoptotic cell death, known as autophagic cell death. Recent studies have shown that sulindac sulfide induces autophagic cell death in human gastric epithelial AGS and rat gastric epithelial RGM-1 cells, and that Survivin down-regulation is involved in this process. Sulindac sulfide treatment increases LC3b-II and APG7 levels and cytosolic vacuole formation, which are indications of autophagy induction. The induced cell death was significantly reduced by pretreatment with the autophagy inhibitors 3-methyladenine and chloroquine, confirming that sulindac sulfide induces autophagic cell death. Furthermore, stable overexpression of Survivin in RGM-1 cells did not inhibit the induction of LC3b-II levels or vacuole formation by sulindac sulfide, but significantly reduced the resulting cell death, suggesting that Survivin may inhibit autophagic cell death downstream of LC3b-II induction and vacuole formation. Depletion of LC3b in AGS cells inhibited the down-regulation of Survivin levels and the induction of cell death by sulindac sulfide, confirming that down-regulation of Survivin occurs in the autophagy pathway downstream of LC3b-II induction by sulindac sulfide. In conclusion, sulindac sulfide induces gastric mucosal injury through the novel mechanism of Survivin-dependent autophagic cell death. These findings could provide new insights into the development of alternative drugs for NSAIDs with a lower risk of gastric mucosal damage. 1
Activities
Sulindac sulfide has shown promising activities in the context of triple-negative breast cancer (TNBC) and colon cancer. In TNBC, Sulindac sulfide has been identified as a potential replacement for g-secretase inhibitors (GSIs) due to its ability to inhibit Notch1 cleavage in TNBC cells, leading to significant inhibition of mammosphere growth across various human and murine TNBC models. Additionally, in a mouse TNBC tumor model, Sulindac sulfide demonstrated remarkable single-agent anti-tumor activity and eliminated Notch1 protein expression in tumors. Importantly, SS did not inhibit Notch cleavage in T-cells, and its anti-tumor effects were enhanced when combined with a-PD1 immunotherapy. In colon cancer, Sulindac sulfide exhibited potent growth inhibitory effects, with sulindac sulfide showing the highest potency among sulindac and its metabolites. Furthermore, Sulindac sulfide downregulated the expression of Sp1, Sp3, and Sp4 proteins, as well as several Sp-regulated genes critical for cancer cell survival, proliferation, and angiogenesis. These findings suggest that SS exerts its antineoplastic effects by targeting the downregulation of Sp transcription factors and Sp-regulated pro-oncogenic gene products. Overall, the pharmacological and immunotherapeutic properties of Sulindac sulfide make it a compelling candidate for further investigation and potential clinical application in the treatment of TNBC and colon cancer. 2
Safety
Sulindac sulfide, a nonsteroidal anti-inflammatory drug, exhibits potential safety concerns that warrant attention. The compound is deemed harmful if ingested and may lead to allergic skin reactions upon contact. Furthermore, inhalation of Sulindac sulfide could induce allergy, asthma symptoms, or breathing difficulties. Moreover, there is suspicion that the substance may harm fertility or pose risks to unborn children. Therefore, caution should be exercised when handling Sulindac sulfide to minimize the risk of exposure and potential adverse effects on both personal health and reproductive wellbeing. 3
Reference
1. Chiou SK, Hoa N, Hodges A. Sulindac sulfide induces autophagic death in gastric epithelial cells via survivin down-regulation: a mechanism of NSAIDs-induced gastric injury. Biochem Pharmacol. 2011 Jun 1;81(11):1317-1323.
2. Li X, Pathi SS, Safe S. Sulindac sulfide inhibits colon cancer cell growth and downregulates specificity protein transcription factors. BMC Cancer. 2015 Dec 16;15:974.
3. 5-Fluoro-2-methyl-1-[[4-(methylthio)phenyl]methylene]-1H-indene-3-acetic acid. European Chemicals Agency, EC / List no. 250-892-2.
Related articles And Qustion
See also

US $1.00/g2024-10-23
- CAS:
- Min. Order:
- 300g
- Purity:
- 99.8%
- Supply Ability:
- 20 TONS