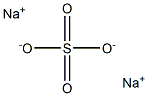
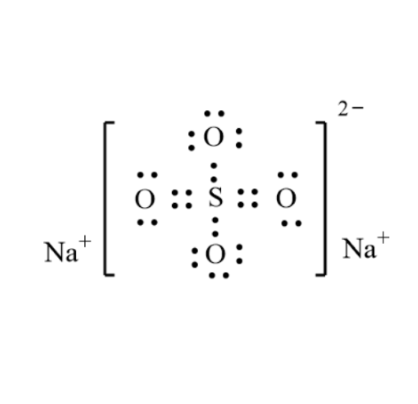
Sodium sulfate(Na2SO4):Lewis structure,Preparation and Uses
Sodium sulfate is the inorganic compound with formula Na2SO4, it is a white solid that are highly soluble in water. With an annual production of 6 million tonnes, the decahydrate is a major commodity chemical product.
Lewis structure
Solubility
Sodium sulfate has unusual solubility characteristics in water. Its solubility in water rises more than tenfold between 0 °C and 32.384 °C, where it reaches a maximum of 49.7 g/100 mL.
Chemical properties
Sodium sulfate is a typical electrostatically bonded ionic sulfate. The existence of free sulfate ions in solution is indicated by the easy formation of insoluble sulfates when these solutions are treated with Ba2+ or Pb2+ salts:
Na2SO4 + BaCl2 → 2 NaCl + BaSO4
The most important chemical sodium sulfate production is during hydrochloric acid production, either from sodium chloride (salt) and sulfuric acid, in the Mannheim process, or from sulfur dioxide in the Hargreaves process. The resulting sodium sulfate from these processes is known as salt cake.
Preparation
Mannheim: 2 NaCl + H2SO4 → 2 HCl + Na2SO4
Hargreaves: 4 NaCl + 2 SO2 + O2 + 2 H2O → 4 HCl + 2 Na2SO4
The second major production of sodium sulfate are the processes where surplus sodium hydroxide is neutralised by sulfuric acid to obtain sulfate (SO2−4) by using copper sulfate (CuSO4) (as historically applied on a large scale in the production of rayon by using copper(II) hydroxide). This method is also a regularly applied and convenient laboratory preparation.
2 NaOH(aq) + H2SO4(aq) → Na2SO4(aq) + 2 H2O(l) ΔH = -112.5 kJ (highly exothermic)
Uses
Sodium sulfate is mainly used as a filler in the manufacture of powdered home laundry detergents and in the Kraft process of paper pulping for making highly alkaline sulfides.
Acute toxicity
LD50 Oral - Rat - female - > 2.000 mg/kg (OECD Test Guideline 423)
Symptoms: Possible damages:, Nausea, Vomiting
LC50 Inhalation - Rat - male and female - 4 h - > 2,4 mg/l - dust/mist
Environmental Fate
Sodium sulfate may persist indefinitely in the environment, but is not likely to show bioaccumulation or food chain contamination effects. If diluted with water and released directly or indirectly into the environment, it is not expected to have a significant impact.
You may like
Related articles And Qustion
See also
Lastest Price from Sodium sulfate manufacturers

US $6.00/kg2025-04-21
- CAS:
- 7757-82-6
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 2000KG/Month

US $0.00-0.00/kg2025-04-21
- CAS:
- 7757-82-6
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 5000kg