Sodium sulfate: Physical Properties and Uses
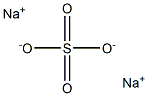
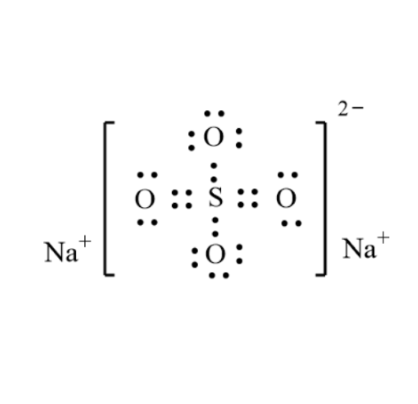
Sodium sulfate is an essential compound of sodium. When anhydrous, it is a white crystalline solid of formula Na2SO4.

Physical Properties
Sodium sulfate has unusual solubility characteristics in water. Its solubility in water rises more than tenfold between 0°C and 32.384°C, where it reaches a maximum of 49.7g/100mL. At this point, the solubility curve changes slope, and the solubility becomes virtually independent of temperature. This temperature of 32.384°C, corresponding to the release of crystal water and melting of the hydrated salt, serves as an accurate temperature reference for thermometer calibration.
Uses
Nowadays, the largest use for sodium sulfate is perhaps as a filler in powdered home laundry detergents. According to studies conducted, total consumption of Na2SO4 in Europe was around 1.6 million tonnes in 2001, of which 80% was used for detergents. However, this use is waning as domestic consumers are increasingly switching to liquid detergents that do not include the chemical.
Another major use for sodium sulfate, particularly in the US, is in the Kraft process for the manufacture of wood pulp. Organics present in the "black liquor" from this process are burnt to produce heat, needed to drive the reduction of sodium sulfate to sodium sulfide. However, this process is being replaced to some extent by newer processes.
What's more, another significant application for sodium sulfate is provided by the glass industry. It is used as a "fining agent", to help remove small air bubbles from molten glass. It also fluxes the glass, and prevents scum formation of the glass melt during refining.
Sodium sulfate is also vital in the manufacture of textiles. It helps in "levelling", reducing negative charges on fibres so that dyes can penetrate evenly. Unlike the alternative sodium chloride, it does not corrode the stainless steel vessels used in dyeing.
Glauber's salt, the decahydrate, was formerly used as a laxative. It has also been proposed for heat storage in passive solar heating systems.6 This takes advantage of the unusual solubility properties, and the elevated heat of crystallisation (78.2 kJ/mol). Additional uses for sodium sulfate include frosting windows, in carpet fresheners, starch manufacture and as an additive to cattle feed. In the laboratory, anhydrous sodium sulfate is widely used as an inert drying agent for organic solutions. Sodium sulfate is added to the solution until the crystals no longer clump together.
You may like
Related articles And Qustion
See also
Lastest Price from Sodium sulfate manufacturers

US $6.00/kg2025-04-21
- CAS:
- 7757-82-6
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 2000KG/Month

US $0.00-0.00/kg2025-04-21
- CAS:
- 7757-82-6
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 5000kg