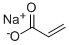Sodium Acrylate & Its Polymers: Cancer Theranostics and Hydrogel Training
Sodium acrylate (SA) is a metal salt prepared by acid-base reaction of sodium hydroxide with acrylic acid. It can be prepared as poly (sodium acrylate) by volumetric polymerization, solution polymerization, emulsion polymerization and suspension polymerization. This will form a water soluble polymer that can be used in a variety of industrial and personal care applications. Sodium acrylate can be used to regulate the particle size of iron oxide particles and has potential applications in bioassays and chemical sensors. It can be used as a reducing and capping agent for gold nanoparticles in biological applications.

Cancer Theranostic Applications of Polyacrylic Acid Nanoplatforms
Polyacrylic acid (PAA), formerly recognized as poly 1-carboxyethylene, is a high molecular weight synthetic (manufactured) polymer made with acrylic acid monomers. Poly (1-carboxyethylene) is a commercialized polymer at a small price. It is a biocompatible superabsorbent polymer soluble in water, nonpoisonous, and recyclable. Superabsorbent polymers (SAP) are hydrophilic net-organized polymers like carboxylic acid, hydroxyl, and amines. Compared to typical water-absorbing polymers, superabsorbents can absorb a high volume of water and eliminate it even under pressure. When all carboxyl groups dissolve, PAA has a high negative charge density. Neutralization transforms the acrylic acid monomer to sodium acrylate monomer in dissolved sodium hydroxide. As a result, this polymer and poly sodium acrylate are among the most widely employed water-soluble anionic polyelectrolytes, such as dispersion compounds, superabsorbent polymers, and ion-exchange resin. As indicated, they are exclusively produced by radical polymerization of sodium (acrylic acid) or acrylic acid. he being safe of PAA-coated materials for biomedical applications should be more scientifically studied. Although PAA has admirable biocompatibility, the toxicity of these NPs for biological organisms should be approved in cell and animal experiments. Additionally, the time and mechanism required for the PAA drug delivery platform for a specific cellular compartment or tissue should be fully addressed.[1]
Comprehensive Training of Poly (Sodium Acrylate) Hydrogel Networks
Hydrogels are soft matters consisting of three-dimensional hydrophilic polymeric networks and confined waters, which have been widely used for diverse applications including wearable electronic and ionotronics devices, soft robotics and machines, tissue engineering, drug delivery,[4] optical fibers, 3D printing materials,[6] and solid-state electrolytes. The design of robust hydrogel networks relies on well understanding of hydrogel topology. Recent researches have demonstrated that some biological-inspired hydrogel networks could interestingly be trained to achieve reverseself-strengthening during repeated exercise. Previously, we showed a simple approach to develop soft poly (sodium acrylate) hydrogels with anti-fracture, super deformability, and harsh-environment insensitivity via hydrophobic homogeneous crosslinking. The poly (sodium acrylate) networks are highly adaptive to be reconstructed by acids or metal ions. Inspired by these ingenious trainable hydrogel networks, we envisioned that a rational remolding strategy of the poly (sodium acrylate) networks would give comprehensive training to reinforce the hydrogels, as well as deepen the understanding of the hydrogel topology.[2]
Herein we proposed a general strategy assisted by strong- acids to enable multiple improvements for hydrogels. Hydrophobic homogeneous cross- linked poly (sodium acrylate)hydrogels were prepared to verify the acid-assisted training strategy. The multiple improvements of the hydrogels was simply achieved by immersing the networks into 4 M H2SO solutions. The superficial poly(sodium acrylate)network transformed to poly (acrylic acid) network after introducing strong-acids, which formed abundant dynamic hydrogen bonding interactions to produce denser topology. The introduction of strong-acids newly generated anti-swelling and self-healing performance of the hydrogels, and enabled mechanical improvement in fracture stress, Young's modulus, and toughness. Following cyclic stretch/release of the pristine acid-containing hydrogels would induce acid infiltration for full transformation of the internal poly (sodium acrylate)network to construct more hydrogen bonding interactions, which further significantly trained the mechanical performance of the hydrogels. The Young's modulus, tensile fracture stress, and toughness of the fully-trained hydrogels were 187.6 times,35.6 times, and 5.4 times enhanced comparing to those of the original hydrogels, respectively.
References
[1]Arkaban H, Barani M, Akbarizadeh MR, Pal Singh Chauhan N, Jadoun S, Dehghani Soltani M, Zarrintaj P. Polyacrylic Acid Nanoplatforms: Antimicrobial, Tissue Engineering, and Cancer Theranostic Applications. Polymers (Basel). 2022 Mar 21;14(6):1259. doi: 10.3390/polym14061259. PMID: 35335590; PMCID: PMC8948866.
[2]Baibin Yang. (2024). Strong Acid Enabled Comprehensive Training of Poly (Sodium Acrylate) Hydrogel Networks. Angewandte Chemie International Edition, 63 34.
You may like
See also
Lastest Price from Sodium acrylate manufacturers
US $0.00/kg2025-10-09
- CAS:
- 7446-81-3
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- Customise

US $79.00-38.00/kg2025-04-21
- CAS:
- 7446-81-3
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 20ton