Application research of divinylbenzene
Introduction
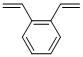
Divinylbenzene (DVB;Figure 1) is a crucial crosslinking agent, with specific applications as follows: serving as a crosslinking component in the copolymerization with styrene, acrylic acid, methacrylic acid, and other vinyl compounds; used for the preparation of polystyrene resins featuring low brittleness, high toughness, and excellent thermal stability; fabricating insoluble ion-exchange resins with low swelling degree; and also applied in the production of resin-dielectrics. This paper mainly focuses on the research examples of its applications.

Hydrophilic Divinylbenzene for Equilibrium Sorption of Emerging Organic Contaminants
Hydrophilic divinylbenzene (DVB) (Bakerbond) has surfaced as a promising sorbent for active sampling of analytes from aqueous matrices over a very broad polarity range. Given this, hydrophilic divinylbenzene may likewise offer potential for passive sampling, if sorbent/water partitioning coefficients ( Ksw) were to be available. In this work, static exposure batch experiments were performed to quantitatively study the equilibrium sorption of 131 environmentally relevant organic contaminants (P values ranging from -1.30 to 9.85) on hydrophilic divinylbenzene. The superior affinity of hydrophilic DVB, as compared to Oasis HLB, for compounds with a broad polarity range was confirmed by functional Fourier-transform infrared spectroscopy and Raman characterization, demonstrating the presence of carboxyl moieties. Concentration effects were studied by increasing compound concentrations in mixture experiments and resulted in the steroidal endocrine disrupting compounds in higher Ksw, while lower Ksw were obtained for the (alkyl)phenols, personal care products, pesticides, pharmaceuticals, and phthalates. Nevertheless, K sw remained constant in the said design for equilibrium water concentrations at environmentally relevant seawater levels. An independent analysis of thermodynamic parameters (change in enthalpy, entropy, and Gibbs free energy) revealed the nature of the main partitioning processes. While polar (log P<4) compounds were mainly served by physisorption, nonpolar (log P>4) compounds also exhibited binding by multiple hydrogen bonding. In conclusion, this research facilitates the future application of hydrophilic DVB for active as well as passive sampling in the analysis of organic contaminants for monitoring purposes and for toxicity testing.[1]
Polydimethylsiloxane/divinylbenzene overcoated fiber
In this study, a comparison of the efficiency of the commercially available polydimethylsiloxane/divinylbenzene (PDMS/DVB) overcoated (OC) fiber used in direct immersion (DI) or in headspace (HS), has been performed by extracting volatiles through solid-phase microextraction (SPME) from a red wine and from a wine model to confirm the results. It was also investigated if a combination of DI followed by HS in a single assay (DI-HS) can provide improvements as compared to the use in DI or in HS only. Furthermore, the use of OC fiber in HS mode was compared with the use of the triphasic phase (TP, in PDMS/CAR/divinylbenzene), known to provide good results in this application. To have information also on fiber specificity, the detected analytes were subdivided into three classes depending on their boiling point. Results show that: OC fiber gives slightly better performance as compared to TP fiber, demonstrating a high efficiency of the OC fiber also in HS mode. Then, comparing the use of the commercial OC fiber in HS, DI and in the combined DI-HS mode, explored for the first time in this study to extract volatiles from wine, the combination DI-HS resulted to provide a more balanced efficiency for all the three groups of analytes, thus being a good compromise when the analytes have a broad range of volatility. Principal component analysis (PCA) and the design of experiment (DoE) were exploited to plan experiments and to help interpreting the results, highlighting that the combined DI-HS approach can be successfully applied to the characterization of wines and of other matrices, where analytes of interest have a wide range of volatility.[2]
Nucleic acids on styrene-divinylbenzene copolymers determined
The introduction of alkylated, nonporous poly-(styrene-divinylbenzene) microparticles in 1992 enabled the subsequent development of denaturing HPLC that has emerged as the most sensitive screening method for mutations to date. Denaturing HPLC has provided unprecedented insight into human origins and prehistoric migrations, accelerated the cloning of genes involved in mono- and polygenic traits, and facilitated the mutational analysis of more than a hundred candidate genes of human disease. A significant step toward increased sample-throughput and information content was accomplished by the recent introduction of monolithic poly(styrene-divinylbenzene) capillary columns. They have enabled the construction of capillary arrays amenable to multiplex analysis of fluorescent dye-labeled nucleic acids by laser-induced fluorescence detection. Hyphenation of denaturing HPLC with electrospray ionization mass spectrometry, on the other hand, has allowed the direct elucidation of the chemical nature of DNA variation and determination of phase of multiple alleles on a chromosome.[3]
Polystyrene-divinylbenzene microspheres applied as absorbents
Hemoperfusion is an important strategy for liver disease treatment. Polystyrene-divinylbenzene (PS-DVB) microspheres are widely applied as absorbents in hemoperfusion to efficiently remove the important toxin bilirubin. However, as another common toxin, endotoxin will remain during this process and cause endotoxemia. Therefore, simultaneous removal of both bilirubin and endotoxin is highly desirable. In the present study, Yang et al. engineered PS-DVB microspheres with polymyxin B sulfate (PMB) to meet this goal. After modification, the novel PMB-engineered (P-PMB) microspheres displayed excellent biocompatibility and hemocompatibility. Notably, compared to PS-DVB microspheres, P-PMB microspheres exhibited markedly stronger detoxification of both bilirubin and endotoxin, increasing by 17.03% and 42.57%, respectively. Overall, we believe that the novel P-PMB microspheres have considerable potential for liver disease treatment in clinical practice.[4]
Divinylbenzene were used to modify other materials
The thermal decomposition product of magnesium hydroxide (MH) is magnesium oxide (MgO), which serves as the foundational material for fireproof layer construction in the condensed phase. However, the weak interaction force between particles of MgO generated by thermal decomposition leads to the insufficient strength and poor adhesion ability of the fireproof layer. The fireproof layer was easily damaged and detached in this study, resulting in the low flame-retardant efficiency of MH. In this work, polycarbosilane (PCS) and divinylbenzene (DVB) were used to modify MH, and EVA/MH/PCS/Divinylbenzene composites were made via melt blending. The flame-retardant properties of EVA/MH/PCS/DVB were evaluated using the limiting oxygen index (LOI), vertical combustion (UL-94), and a cone calorimeter (CONE). The thermal stability of the composites and flame retardants was analyzed using a thermogravimetric analyzer. The char layer structure was observed and analyzed using scanning electron microscopy (SEM) and X-ray photoelectron spectroscopy (XPS), respectively. The results indicate that the LOI of the EVA/MH/PCS/Divinylbenzene with 50 wt.% flame retardants in total was as high as 65.1, which increased by 160% in comparison with EVA/MH. Furthermore, the total smoke production (TSP) of the EVA/MH/PCS/Divinylbenzene composite decreased by 22.7% compared to EVA/MH/PCS; the thermal stability of the MH/PCS/DVB and EVA/MH/PCS/DVB improved to some extent; and the compact residual char after the combustion of EVA/MH/PCS/DVB had fewer cracks due to the adhesive effect induced by PCS/Divinylbenzene.[5]
References
[1] Huysman S, Vanryckeghem F, De Paepe E, et al. Hydrophilic Divinylbenzene for Equilibrium Sorption of Emerging Organic Contaminants in Aquatic Matrices. Environ Sci Technol. 2019;53(18):10803-10812. doi:10.1021/acs.est.9b01814
[2] Lenti L, Scortichini S, Pacetti D, Cespi M, Fiorini D. Polydimethylsiloxane/divinylbenzene overcoated fiber and its application to extract and analyse wine volatile compounds by solid-phase microextraction and gas chromatography coupled to mass spectrometry: direct immersion, headspace or both?. Food Res Int. 2021;148:110632. doi:10.1016/j.foodres.2021.110632
[3] Oefner PJ, Huber CG. A decade of high-resolution liquid chromatography of nucleic acids on styrene-divinylbenzene copolymers. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;782(1-2):27-55. doi:10.1016/s1570-0232(02)00700-6
[4] Yang K, Peng Y, Wang L, Ren L. Polymyxin B engineered polystyrene-divinylbenzene microspheres for the adsorption of bilirubin and endotoxin. RSC Adv. 2021;11(63):39978-39984. Published 2021 Dec 15. doi:10.1039/d1ra06684f
[5] Li S, Wang C, Wang G, Wang Y, Han Z. Polycarbosilane/Divinylbenzene-Modified Magnesium Hydroxide to Enhance the Flame Retardancy of Ethylene-Vinyl Acetate Copolymer. Polymers (Basel). 2023;15(22):4440. Published 2023 Nov 17. doi:10.3390/polym15224440
You may like
Lastest Price from Divinylbenzene manufacturers

US $0.00-0.00/KG2025-04-15
- CAS:
- 1321-74-0
- Min. Order:
- 50KG
- Purity:
- 99%
- Supply Ability:
- 500000kg

US $100.00/kg2025-04-15
- CAS:
- 1321-74-0
- Min. Order:
- 1kg
- Purity:
- 99
- Supply Ability:
- 5000
![1760-24-3 N-[3-(Trimethoxysilyl)propyl]ethylenediamineCarbon nanotubesApplication](/NewsImg/2025-11-29/6390005339737009573027011.jpg)
