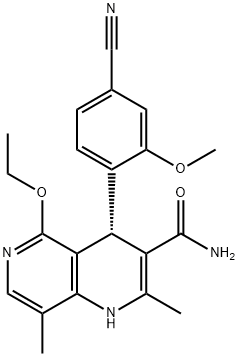
Side effects of Finerenone in the treatment of diabetic nephropathy
Introduction of Finerenone
Finerenone is a novel non-steroidal, highly selective salicorticoid receptor antagonist (MRA) with high binding affinity, high MR selectivity and short plasma half-life. It is approved for the treatment of chronic kidney disease (CKD) in patients with type 2 diabetes mellitus (T2D). finerenone has been shown to improve multiple pathophysiological parameters in heart failure with preserved ejection fraction (HFpEF), with potentially beneficial effects.
Finerenone and Diabetic Nephropathy
Finerenone is a non-steroidal selective hydrocorticoid receptor antagonist. Studies have shown that Finerenone has more potent anti-inflammatory and antifibrotic effects than the steroidal salicorticoid receptor antagonists, reducing albuminuria in short-term trials involving patients with chronic kidney disease (CKD) and type 2 diabetes mellitus, and reducing the urinary albumin/creatinine ratio in patients with CKD treated with a RAS blocker, while having a lesser effect on serum potassium levels than spironolactone.
Safety of Finerenone
In short-term studies in patients with CKD reduced ejection heart failure, with or without T2D, finerenone 20 mg appears to have a better renal outcome compared with spironolactone a better mortality outcome compared with eplerenone, with significantly lesser hyperkalemia compared to both spironolactone finerenone. In long-term studies in patients with CKD T2D, finerenone 10/20 mg significantly reduces progression renal disease reduced CV endpoints (especially heart failure hospitalization) compared to placebo. Finerenone has no effect HbA1c, body weight, sexual side effects including gynecomastia, has only a modest effect blood pressure. However, hyperkalemia leading to drug withdrawal was significantly higher with finerenone compared to placebo.
Findings in a study report of major adverse events after the use of fenugreek in patients with type 2 diabetes mellitus:finerenone increased risk for hyperkalemia by two times compared to placebo (RR = 2.03, 95% CI;1.82–2.26, I2 = 0%, p<0.00001), while it also significantly increased risk for hospitalization due to hyperkalemia (RR = 5.86, 95% CI 2.99 – 11.47, I2 = 0%, p<0.00001). We have also documented that finerenone did not significantly affect risk for development AKI (p = 0.94) or hospitalization in context AKI (p = 0.92). In addition, finerenone resulted in a marginally non-significant reduction in risk for all-cause death, equal to 10% (p = 0.05). However, extra caution is required, since hyperkalemia appears to be most important side effect with use finerenone, representing also main cause hospitalization due to a drug side effect.
References:
[1] D. PATOULIAS. MAJOR ADVERSE EVENTS WITH THE USE OF FINERENONE IN PATIENTS WITH TYPE 2 DIABETES MELLITUS: A META-ANALYSIS OF CARDIOVASCULAR OUTCOME TRIALS[J]. Journal of Hypertension, 2022, 40 1: e158-e159. DOI:10.1097/01.hjh.0000836952.24656.9c.
[2] AWADHESH KUMAR SINGH. Finerenone in diabetic kidney disease: A systematic review and critical appraisal.[J]. Diabetes and Metabolic Syndrome-Clinical Research and Reviews, 2022, 16 10: 102638. DOI:10.1016/j.dsx.2022.102638.
You may like
Related articles And Qustion
See also
US $0.00/kg2025-12-11
- CAS:
- 1050477-31-0
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10000KGS

US $5.00-0.50/KG2025-05-30
- CAS:
- 1050477-31-0
- Min. Order:
- 1KG
- Purity:
- 99% hplc
- Supply Ability:
- 500TONS