Seven steps to synthesize Peficitinib hydrobromide
Description
Peficitinib is a Janus kinase (JAK)1, JAK2, JAK3, and tyrosine kinase (Tyk)2 (pan-JAK) inhibitor recently approved in Japan for the treatment of rheumatoid arthritis. Inhibition of JAK suppresses the activation of cytokine signaling pathways involved in inflammation and joint destruction in rheumatoid arthritis[1].
Biological Action
The drug inhibits JAK1, JAK2, JAK3, and tyrosine kinase 2 (TYK2) enzymes with moderate selectivity for JAK3. Inhibition of JAK enzymes suppresses the activation of cytokine-mediated signal transduction pathways responsible for inflammation and joint destruction and contribute to RA. Peficitinib hydrobromide inhibits signal inducer and activator of transcription 5 (STAT5) phosphorylation, a marker of JAK inhibition in human whole blood.
Synthesis method
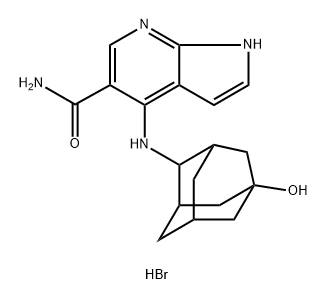
The seven-step longest linear synthetic sequence commenced with 4-chloro-7- azaindole (178), which was initially N-protected with triisopropylsilyl chloride (TIPSCl) to give 179 (shown below)[2]. When 179 was treated with sec-BuLi, the TIPS group was critical in blocking C-2 and directing lithiation to C-5. An ethyl chloroformate quench yielded the desired C-5-substituted ethyl ester, deprotected with TBAF to provide 7- azaindole 180. Basic hydrolysis of the ester in 180 and subsequent treatment with CDI/aqueous NH4OH provided amide 181 in 82% yield over the two-step sequence.
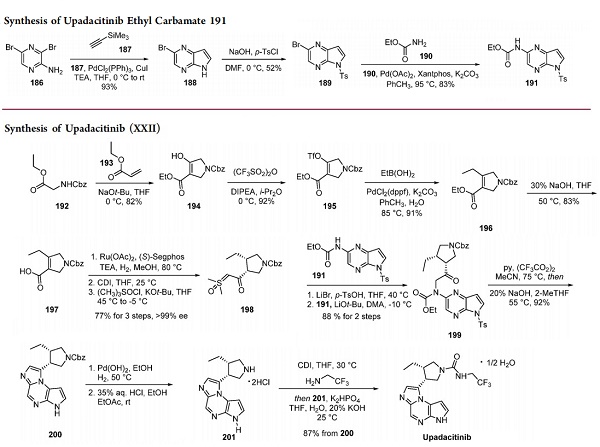
Completion of the synthesis of the drug took place, as depicted in the above figure. The desired trans-4-aminoadamantan- 1-ol (185) was obtained from a diastereomeric mixture of 4- aminoadamantan-1-ol (182) (1:1 dr) in two steps. First, the amine moiety in 182 was protected with benzyl chloroformate, and the resulting diastereomers (183 and 184) were chromatographically separated. The desired trans isomer 184 was subsequently treated with palladium on carbon in hydrogen gas to afford 185. Subjection of 185 to chloropyridine 181 at forcing temperatures (200 °C) furnished the peficitinib free base, which was converted to the HBr salt of peficitinib, enabling isolation of the drug in crystalline form.
References
[1] Anthony Markham, Susan J Keam. “Peficitinib: First Global Approval.” Drugs 79 8 (2019): 887–891.
[2] Andrew C. Flick. “Synthetic Approaches to the New Drugs Approved during 2019.” Journal of Medicinal Chemistry 64 7 (2021): 3604–3657.
See also
US $0.00/kg2025-11-12
- CAS:
- 1353219-05-2
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1000kg

US $0.00/g2025-09-10
- CAS:
- 1353219-05-2
- Min. Order:
- 1g
- Purity:
- More Than 99%
- Supply Ability:
- 100kg/Month