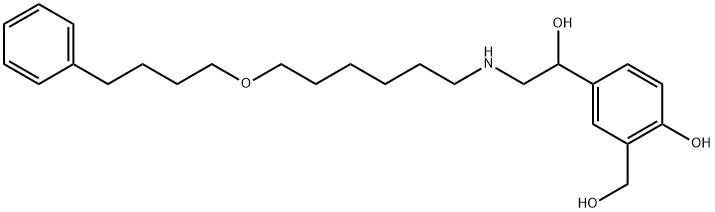
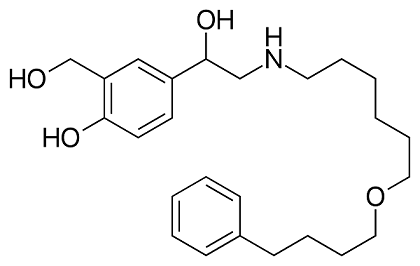
Salmeterol: pharmacological properties, therapeutic efficacy and safety
General Description
Salmeterol is a selective long-acting beta2-agonist that primarily acts by causing bronchodilation and inhibiting inflammatory responses in the airways. When combined with fluticasone propionate, it provides better asthma control and improves lung function compared to fluticasone propionate alone. This combination therapy has a corticosteroid-sparing effect and has shown significant improvements in asthma-related quality of life. Salmeterol/fluticasone propionate is generally safe and well-tolerated, with common adverse effects including upper respiratory tract infection, pharyngitis, headaches, and throat irritation. Serious events are rare with this treatment. Overall, salmeterol/fluticasone propionate is an effective and safe option for managing asthma symptoms in both adults and children.
Figure 1. Aerosol of salmeterol
Pharmacological properties
Salmeterol is a selective long-acting beta2-agonist (LABA) with pharmacological properties that contribute to its therapeutic effects. It primarily acts by causing bronchodilation, which helps to relax the smooth muscles in the airways and improve airflow. Additionally, salmeterol inhibits the release of hypersensitivity mediators from mast cells, reducing the inflammatory response associated with bronchial hyperreactivity. The pharmacokinetics of salmeterol and fluticasone propionate, when given concurrently, are generally similar to their individual administration. There is no significant systemic pharmacokinetic interaction between the two drugs. It is important to note that the action of salmeterol and fluticasone propionate is mainly localized to the lung, and plasma levels do not necessarily reflect therapeutic effectiveness. Salmeterol undergoes extensive metabolism through hydroxylation, while fluticasone propionate is primarily metabolized into an inactive metabolite by the cytochrome P450 (CYP) isoenzyme CYP3A4. Overall, the pharmacological properties of salmeterol, both as a LABA and in combination with fluticasone propionate, contribute to its efficacy in managing respiratory conditions such as asthma and chronic obstructive pulmonary disease. 1
Therapeutic efficacy
Salmeterol is a medication commonly used for the treatment of asthma. It is often combined with fluticasone propionate to create a maintenance therapy that has been found to improve lung function and asthma symptoms to a greater extent than other treatments. Numerous well-designed, long-term studies, lasting up to 1 year, have consistently shown that twice-daily salmeterol/fluticasone propionate provides better asthma control than twice-daily fluticasone propionate alone. This combination therapy also has a corticosteroid-sparing effect, meaning that a lower dose can achieve a similar level of control compared to a higher dose of fluticasone propionate alone. Furthermore, studies comparing salmeterol/fluticasone propionate with formoterol/budesonide maintenance therapy have yielded mixed results. Some studies indicate similar efficacy between the two agents, while others suggest that salmeterol/fluticasone propionate may be more effective. It is worth noting that salmeterol/fluticasone propionate has shown significant improvements in health-related quality of life (HR-QOL) for asthma sufferers. Asthma quality-of-life questionnaire scores have improved more with salmeterol/fluticasone propionate compared to fluticasone propionate alone or oral montelukast. However, the results of HR-QOL studies comparing salmeterol/fluticasone propionate with formoterol/budesonide have been inconclusive. Overall, salmeterol/fluticasone propionate maintenance therapy has proven to be a highly effective option for managing asthma symptoms, improving lung function, and enhancing quality of life for patients across different age groups. 2
Safety
Salmeterol, in combination with fluticasone propionate, is generally safe and well-tolerated in adults, adolescents, and children. The most common treatment-related adverse effects reported across various studies include upper respiratory tract infection, pharyngitis, headaches, and throat irritation/cough. These effects are generally similar to those observed with other comparators like fluticasone propionate alone, montelukast, or formoterol/budesonide. In a carefully designed 12-week study involving asthmatic children aged 6-14 years, the most frequently reported adverse event associated with salmeterol/fluticasone propionate or oral montelukast was headache. The incidence of treatment-related adverse effects was found to be 2% in both treatment groups. Serious events were reported in zero patients in the salmeterol/fluticasone propionate group, while three patients experienced serious events in the oral montelukast group. Furthermore, in children aged 4-11 years, a study evaluating urinary cortisol excretion over a 24-hour period found that it remained within normal limits after 12 weeks of treatment with salmeterol/fluticasone propionate 50 μg/100 mg. Overall, these findings suggest that salmeterol, in combination with fluticasone propionate, is well tolerated with an acceptable safety profile in both pediatric and adult populations. 3
Reference
1. Reynolds NA, Lyseng-Williamson KA, Wiseman LR. Inpropionate: a review of its haled salmeterol/fluticasone use in asthma. Drugs. 2005; 65(12): 1715-1734.
2. McKeage K, Keam SJ. Salmeterol/fluticasone propionate: a review of its use in asthma. Drugs. 2009; 69(13): 1799-1828.
3. Maspero J, Guerra F, Cuevas F, et al. Efficacy and tolerability of salmeterol/fluticasone propionate versus montelukast in childhood asthma: a prospective, randomized, double-blind, double-dummy, parallel-group study. Clin Ther. 2008; 30(8): 1492-1504.
You may like
Related articles And Qustion
See also
Lastest Price from Salmeterol manufacturers

US $55.00-550.00/kg2024-08-27
- CAS:
- 89365-50-4
- Min. Order:
- 10kg
- Purity:
- 0.99
- Supply Ability:
- 20tons

US $0.00-0.00/kg2022-11-15
- CAS:
- 89365-50-4
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 100000000000