Salcaprozate Sodium Facilitates Oral Delivery of Low-Permeability Drugs
General Description
Salcaprozate sodium is a carrier molecule that enhances the absorption of active pharmaceutical ingredients (APIs) in oral formulations. Its exact mechanism of action is not fully understood, but it may increase the permeability of gastrointestinal epithelial cells or form transportable complexes with APIs. Although there is ongoing debate and no direct evidence for these hypotheses, Salcaprozate sodium's ability to enhance API absorption offers potential benefits for drug efficacy and bioavailability. It has been studied extensively in oral formulations, particularly for low-permeability drugs like heparin. Safety studies in both preclinical and clinical settings have demonstrated its tolerability, supporting its designation as a Generally Recognized As Safe additive by the FDA.

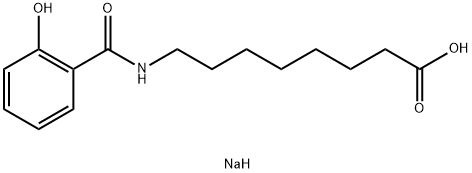
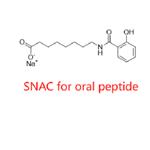
Figure 1. Salcaprozate sodium
Mechanism of action
Salcaprozate sodium is a carrier molecule that has been shown to enhance the absorption of active pharmaceutical ingredients, although the exact mechanism by which it works is not yet fully understood. One theory suggests that Salcaprozate sodium increases the membrane permeability of gastrointestinal epithelial cells, possibly through membrane perturbation, membrane fluidization, changes in API solubility, or opening of tight junctions. Another hypothesis proposes that Salcaprozate sodium forms a transportable complex with APIs through non-covalent bonding, exposing more hydrophobic regions of the API molecule and facilitating its transcellular transport. While there is no direct evidence supporting these hypotheses, they remain under debate. For example, it is unclear whether Salcaprozate sodium's permeation-enhancing effect is related to the cell membrane perturbation effects induced by surfactants. Additionally, questions about the thermodynamics of non-covalent binding between Salcaprozate sodium and APIs and the role of transcellular transport in Salcaprozate sodium's action remain unresolved. Caco-2 cell model studies suggest that Salcaprozate sodium's permeation-enhancing effect may not be solely attributed to transcellular transport. Nonetheless, Salcaprozate sodium's ability to enhance API absorption provides a promising avenue for improving drug efficacy and bioavailability. 1
Applications in oral formulations
Salcaprozate sodium has been extensively studied for its application in oral formulations of low-permeability active drugs since the late 1990s. In a Phase I trial conducted in 1998, a combination of 2.25g Salcaprozate sodium and 30,000-150,000IU heparin was administered orally, resulting in increased anticoagulant activity, as indicated by prolonged activated partial thromboplastin time (aPTT) and generation of anti-Xa factor. Subsequent Phase I and II trials were conducted on patients undergoing total hip replacement, with oral heparin doses of 60,000IU (+1.5g Salcaprozate sodium) or 90,000IU (+2.25g Salcaprozate sodium). The results showed that oral heparin induced similar anti-Xa factor activity compared to subcutaneous injection of heparin (5,000IU) and demonstrated comparable major bleeding events to the subcutaneous group, supporting the safety of oral heparin administration. However, in the Phase III trial (PROTECT study) in 2002, oral heparin did not meet its primary endpoint of superior efficacy over subcutaneous heparin in preventing lower limb venous thrombosis by day 27-30. Due to poor patient compliance caused by the bitter taste of the oral solution, further development was halted. Subsequently, an oral heparin and Salcaprozate sodium soft gel capsule formulation (75,000IU heparin/500mg Salcaprozate sodium) was developed, and a pharmacokinetic-pharmacodynamic study in 2007 confirmed its prolonged aPTT effect. However, it was ultimately abandoned due to failure to meet the endpoints of Phase III clinical trials. 2
Safety
Salcaprozate sodium has been extensively studied for its safety in both preclinical and clinical studies. In preclinical studies, NOAEL was established as 1 g/kg/day in rats for subchronic oral administration, and no significant adverse effects were observed in pregnant rats at doses up to 1 g/kg/day. In monkey studies, gastrointestinal effects such as vomiting and diarrhea were observed at doses ≥1.8 g/kg/day. In clinical studies, Salcaprozate sodium was found to be safe in patients with varying degrees of impaired kidney and liver function, indicating that the dose of Salcaprozate sodium in pharmaceutical products is safe for human consumption. Furthermore, based on these safety data, Salcaprozate sodium has been designated as a Generally Recognized As Safe (GRAS) additive by the US Food and Drug Administration. Overall, these studies provide a strong foundation for the further development of Salcaprozate sodium-based oral pharmaceutical products, ensuring their safety for use in patients. 3
Reference
1. Twarog C, Fattah S, Heade J, Maher S, Fattal E, Brayden DJ. Intestinal Permeation Enhancers for Oral Delivery of Macromolecules: A Comparison between Salcaprozate Sodium (SNAC) and Sodium Caprate (C10). Pharmaceutics. 2019 Feb 13;11(2):78.
2. Mousa SA, Zhang F, Aljada A, Chaturvedi S, Takieddin M, Zhang H, Chi L, Castelli MC, Friedman K, Goldberg MM, Linhardt RJ. Pharmacokinetics and pharmacodynamics of oral heparin solid dosage form in healthy human subjects. J Clin Pharmacol. 2007 Dec;47(12):1508-1520.
3. McCartney F, Gleeson JP, Brayden DJ. Safety concerns over the use of intestinal permeation enhancers: A mini-review. Tissue Barriers. 2016 Apr 12;4(2):e1176822.
You may like
Related articles And Qustion
See also
Lastest Price from Salcaprozate Sodium manufacturers
US $0.00/g2025-11-05
- CAS:
- 203787-91-1
- Min. Order:
- 10g
- Purity:
- 0.99
- Supply Ability:
- /
US $1.00-4.00/KG2025-09-02
- CAS:
- 203787-91-1
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 200000KG