Ruthenium(III) Chloride: A Key Catalyst in Modern Chemistry and Advanced Materials
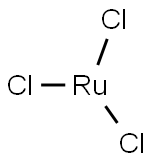
Ruthenium(III) chloride (RuCl₃) has become a very important compound in chemistry today, especially because of how useful it is in speeding up chemical reactions and helping to create advanced materials. Most often, it's found in a form that includes water (hydrated RuCl₃•xH₂O), and this particular transition metal compound stands out for being flexible, stable, and highly useful in a variety of chemical processes.

Figure 1 Characteristics of Ruthenium(III) chloride
Structure and Chemical Properties
Ruthenium is part of the platinum group of metals, which are well-known for their powerful ability to act as catalysts—substances that speed up chemical reactions without being used up themselves. In its Ruthenium(III) chloride form, ruthenium takes on a +3 oxidation state, meaning it tends to lose three electrons during reactions. This state helps make it both reactive and stable at the same time, which is ideal for use in many types of synthetic processes.
When Ruthenium(III) chloride doesn't have any water attached to it (anhydrous form), it forms a hexagonal crystal structure. However, it's more commonly found as a dark brown or black powder in its hydrated state. This powdery form makes it easy to use in different reactions and applications.
Catalytic Uses
Ruthenium(III) chloride is primarily known for its role as a catalyst in a wide range of chemical reactions, especially in organic chemistry. It plays a big part in reactions like hydrogenation (adding hydrogen), oxidation (removing electrons), and metathesis (swapping chemical groups between molecules).
Hydrogenation: Ruthenium(III) chloride is particularly good at helping to add hydrogen to organic compounds, such as alkenes, alkynes, and carbonyl groups. It often works under less intense conditions than other catalysts, which makes it a popular choice for selective hydrogenation, where only certain parts of a molecule are modified.
Oxidation: Ruthenium(III) chloride is also effective in oxidation reactions, such as converting alcohols into aldehydes or ketones. In these reactions, special ruthenium-containing compounds called "oxo species" help transfer electrons, which speeds up the process. Ruthenium(III) chloride’s efficiency in these reactions has made it useful even in large-scale industrial processes, such as those used in pharmaceutical manufacturing, where its high performance is highly valued.
Metathesis: Another important role of Ruthenium(III) chloride is in olefin metathesis, a reaction that forms carbon-carbon double bonds and is crucial in both polymer chemistry and organic synthesis. Ruthenium(III) chloride is used to make ruthenium-based catalysts, like the well-known Grubbs catalysts, which help with important reactions like ring-closing metathesis and cross-metathesis.
New and Emerging Uses: Beyond Catalysis
While Ruthenium(III) chloride is famous for its catalytic abilities, researchers are finding more ways to use it, especially in materials science. It is being used to create new ruthenium-based nanomaterials and thin films, which are important in areas like electronics, sensors, and fuel cells. The compound’s excellent conductivity and catalytic properties make it highly valuable in these advanced technologies.
For instance, carefully breaking down Ruthenium(III) chloride can produce ruthenium nanoparticles, which have potential applications in these high-tech areas.
Additionally, Ruthenium(III) chloride has drawn interest in the field of spintronics, which is an area of study focused on the magnetic properties of materials and their potential use in technologies like quantum computing. The magnetic structure of Ruthenium(III) chloride has unusual properties, and researchers are exploring how it might be used to study quantum spin liquids, a strange state of matter that could open up new possibilities in condensed matter physics and future technology.
Environmental and Economic Considerations
While Ruthenium(III) chloride is incredibly useful, producing ruthenium and converting it into Ruthenium(III) chloride is expensive and can have environmental impacts. Ruthenium is a rare metal, usually found alongside platinum and palladium, and its limited availability drives up costs. This can make it harder to use on a larger scale.
However, researchers are working on ways to make ruthenium-based processes more sustainable, such as improving recycling methods and designing catalysts that require less of the metal. These advances could help make Ruthenium(III) chloride and other ruthenium compounds more accessible and environmentally friendly.
References:
[1] ROBERT E. CONNICK D A F. Ruthenium(III) Chloride Complexes: RuCl2+[J]. Journal of the American Chemical Society, 1960, 82 16: 4123-4440. DOI:10.1021/ja01501a018.[2] M. M. TAQUI KHAN A P R G Ramachandraiah. Ruthenium(III) chloride in aqueous solution: electrochemical and spectral studies[J]. Inorganic Chemistry, 1986, 25 5: 589-714. DOI:10.1021/ic00225a015.
Lastest Price from Ruthenium(III) chloride manufacturers

US $10.00/KG2025-04-21
- CAS:
- 10049-08-8
- Min. Order:
- 100KG
- Purity:
- 99%
- Supply Ability:
- 100 mt

US $990.00-800.00/ton2025-04-21
- CAS:
- 10049-08-8
- Min. Order:
- 1ton
- Purity:
- 99%
- Supply Ability:
- 5000