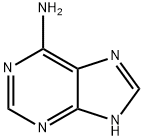
Role of adenine functional groups
The AG dinucleotide at the 3′ splice sites of metazoan nuclear pre-mRNAs plays a critical role in catalytic step II of the splicing reaction. Previous studies have shown that replacement of the guanine by adenine in the AG (AG → GG) inhibits this step. We find that the second step was even more severely inhibited by cytosine (AG → CG) or uracil (AG → UG) substitutions at this position. By contrast, a relatively moderate inhibition was observed with a hypoxanthine substitution (AG → HG). When adenine was replaced by a purine base (AG → PG) or by 7-deazaadenine (AG → c7AG), little effect on the second step was observed, suggesting that the 6-NH2 and N7 groups do not play a critical role in adenine recognition. Finally, replacement of adenine by 2-aminopurine (AG → 2-APG) had no effect on the second step. Taken together, our results suggest that the N1 group of adenine functions as an essential determinant in adenine recognition during the second step of pre-mRNA splicing.
The accurate removal of introns from metazoan pre-mRNAs proceeds by a two-step mechanism that involves two in-line transesterification reactions. The first step of splicing is initiated by a nucleophilic attack by the 2′-hydroxyl group of the branchpoint adenosine on the phosphodiester bond of the guanine residue at the 5′ end of the intron. This step results in the generation of a lariat intermediate containing a 2′-5′ phosphodiester bond and the free 5′ exon. In the second step, the 3′-hydroxyl group at the terminus of the 5′ exon attacks the phosphodiester bond of the 3′ splice site, resulting in ligation of the two exons and the concomitant release of the lariat intron (1–4).
The splicing reaction takes place within the spliceosome, a 50–60S ribonucleoprotein complex consisting of five small nuclear RNAs (snRNAs), U1, U2, U4, U5, and U6, and more than 50 distinct proteins. Spliceosome assembly involves the formation of four discrete complexes on the pre-mRNA, in the order E, A, B, and C (5–7). Commitment of pre-mRNA to the splicing pathway takes place in the E (early) complex, which contains U1 small nuclear ribonucleoprotein (snRNP) as well as several non-snRNP proteins, including the essential splicing factor U2AF (8–11). The binding of U2 snRNP to the branchpoint sequence leads to the formation of complex A, whereas the subsequent entry of U4/U6·U5 tri-snRNP generates complex B. Finally, a series of conformational changes that dislodge U1 and U4 snRNPs creates the catalytically active spliceosome complex C (12).
In higher eukaryotes, three distinct intron sequences are required for spliceosome assembly and pre-mRNA splicing: the 5′ splice site, the branchpoint sequence, and the 3′ splice site, (/GURAGY, YNYURAC, and YAG/, respectively; where a slash (/) denotes a splice site; N denotes any nucleotide, R denotes purine, and Y denotes pyrimidine; underlining indicates the conserved nucleotides). Genetic and biochemical analyses suggest that a number of RNA–RNA interactions involving snRNAs and these sequences are responsible for the selection of the correct chemical groups for both steps of the splicing reaction (12–14). For the first step, the U1 snRNA/5′ splice site (15–17) and the U2 snRNA/branchpoint interactions (18–20) define, at least in part, the 5′ splice site and the attacking nucleophile, respectively. However, the correct 5′ splice site appears to be established by exchanging the U1 snRNA/5′-splice-site interaction for interactions with U5 and U6 snRNAs (21–24). In contrast to the information available on the recognition and selection of the 5′ splice site and the branchpoint sequence, relatively little is known about the 3′-splice-site AG selection during the second step of splicing. In both yeast and mammals, the 3′ splice site consists of a highly conserved sequence element, YAG, and the pre-mRNA is cleaved after the G residue (25). Some progress has been made in the identification of the RNA and protein factors that are required for 3′-splice-site AG recognition in yeast and mammals. In addition, a number of models have been proposed for the selection of the correct AG dinucleotide during catalytic step II of pre-mRNA splicing (12, 25). Most significantly, a non-Watson–Crick base-pairing interaction between the first and the last residues of introns is thought to be important for the positioning of the 3′ splice site for the second step of the splicing reaction (26, 27).
Materials and Methods
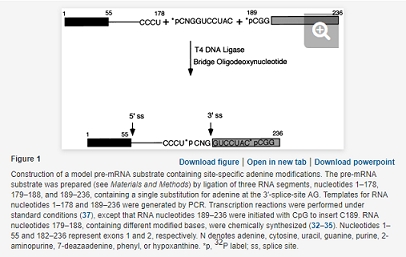
Plasmid pPIP85.B-RG, a derivative of plasmid pPIP85.B (31), was constructed by subcloning the reverse-transcribed cDNA of the three-part ligation product RNA, nucleotides 1–178, 179–188, and 189–236 (Fig. 1). The nucleotide sequence, base pairs 189–234 of pPIP85.B, was replaced by 5′-CGGCAACATACTGCAGGACAAACTCTTCGCGGTCTCTGCATGCAAGCT-3′. Plasmid pPIP85.B-RG encodes the following RNA sequence: 5′-GGGCGAAUUCGAGCUCACUCUCUUCCGCAUCGCUGUCUGCGAGGUACCCUACCAG/GUGAGUAUGGAUCCCUCUAAAAGCGGGCAUGACUUCUAGAGUAGUCCAGGGUUUCCGAGGGUUUCCGUCGACGAUGUCAGCUCGUCUCGAGGGUGCUGACUGGCUUCUUCUCUCUUUUUCCCUCAG/GUCCUACCGGCAACAUACUGCAGGACAAACUCUUCGCGGUCUCUGCAUGCAAGCU-3′. Slashes represent splice sites and the underlined A indicates the preferred branchpoint.
Chemical Synthesis of Modified Oligoribonucleotides.
Modified phosphoramidites for inosine (32), purine riboside (33), 2-aminopurine (2-AP) riboside (34), 7-deazaadenosine (c7A) (33), and phenylriboside (35) were synthesized according to the published protocol, except that the acetyl group was used for protection of 2-NH2 functionality in the 2-AP riboside. These monomers were introduced into oligoribonucleotides by using standard coupling cycles, and the assembled oligonucleotides were purified by HPLC, as described earlier (36).
In vitro RNA Transcription and Construction of Pre-mRNA Substrates.
Plasmid pPIP85.B-RG was used to transcribe pre-mRNA substrates in vitro. Two DNA templates were synthesized by PCR from the plasmid pPIP85.B-RG. The upstream DNA template encodes the sequence of the 5′ exon and the entire intron, except for the CAG sequence of the 3′ splice site. The 5′ primer for making the upstream DNA template was 5′-TAATACGACTCACTATAGGG-3′, and the 3′ primer was 5′-AGGGAAAAAGAGAGAAGAAG-3′. The downstream template encodes the entire exon 2, except for the first 7 nucleotides. The 5′ primer for making the downstream DNA template was 5′-TAATACGACTCACTATAGGCAACATACTGCAGG-3′ and the 3′ primer was 5′-AGCTTGCATGCAGAGACCGC-3′. The resulting PCR-generated DNA templates are under the control of the T7 RNA polymerase promoter.
The RNAs (nucleotides 1–178 and 189–236) were transcribed with upstream and downstream DNA templates by following standard protocols (37). Transcription of RNA (nucleotides 189–236) was initiated with CpG dinucleotide (Sigma) to insert C189. The full length pre-mRNA substrates were prepared by ligating three RNA segments (nucleotides 1–178, 179–188, and 189–236), containing a single substitution at the adenosine of the 3′-splice-site AG (position N180; see Fig. 1), where N denotes adenine, cytosine, guanine, uracil, purine, 2-AP, c7A, hypoxanthine, or phenyl. After end-labeling with [γ-32P]ATP and polynucleotide kinase, RNA nucleotides 179–188 and 189–236, together with 5′-capped [m7G(5′)ppp(5′)G] RNA nucleotides 1–178, were annealed to a complementary oligonucleotide and ligated, by using T4 DNA ligase (38). The ligated product was purified on 8% polyacrylamide (29:1)/8 M urea gels, run in 1× Tris–borate/EDTA buffer; the pre-mRNA was isolated by overnight elution, followed by ethanol precipitation.
Nuclear Extracts.
Nuclear extracts were prepared from HeLa cells, essentially as described by Dignam et al. (39).
In Vitro Splicing Assay.
High specific activity 32P-labeled pre-mRNAs, labeled within the intron and exon 2, were incubated under standard splicing conditions (40), with 50% vol/vol HeLa cell nuclear extract. Reactions (25 μl) were incubated at 30°C for the times indicated above each lane (Figs. 3–5). The products of the splicing reactions were separated on 13% polyacrylamide (29:1)/8 M urea gels run in 1× Tris–borate/EDTA buffer. Polyacrylamide gels were quantified with a Molecular Dynamics PhosphorImager (Fuji) and MACBAS software, version 2.5. For each lane, an individual background value was determined, and the relative amount of RNA in each band was expressed as a percentage of the total, which was obtained by summing the values of the pre-mRNA, lariats, and product at that time point.
Results
Experimental Design.
Previous studies (28–30) have shown that an AG → GG mutation at the 3′ splice site of mammalian pre-mRNAs does not have a significant effect on the efficiency of the first step of splicing if the substrate contains a functional branchpoint sequence, followed by a pyrimidine tract. However, the AG → GG mutation severely inhibited the second step of pre-mRNA splicing. In addition, a pre-mRNA, lacking the 3′-splice-site AG and the downstream exon, can undergo the first step of the splicing reaction: cleavage at the 5′ splice site and lariat formation (29). Thus, catalytic step II specifically requires an adenine base, or the functional group(s) of guanine is inhibitory. These studies also demonstrated that the sequences required for the second step of the splicing reaction, i.e., the lariat release and the exon ligation, could be studied without interfering with the first step chemistry.
To investigate the role of the 3′-splice-site adenine in catalytic step II, we carried out two sets of experiments: (i) adenine was replaced by a pyrimidine base (cytosine or uracil), and (ii) adenine was replaced by several purine-based nucleotides. The requirement of a purine ring for step II chemistry can be tested by replacing the adenine with cytosine or uracil, whereas the replacement of adenine by purine-derived nucleotides allows evaluation of the role of different functional group(s) of adenine during the second step of splicing. Finally, the replacement of adenine by a phenyl group tests the requirement of a conventional base for the second step of splicing.
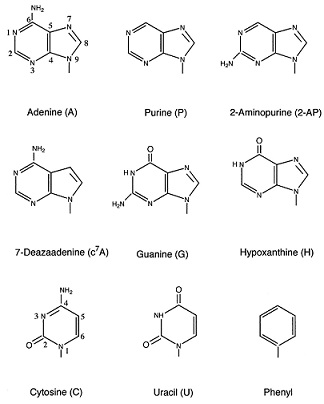
To analyze the role of adenine functional groups during the second step of splicing, the 3′-splice-site adenine was replaced by a number of purine-derived bases: purine, which does not have a functional group at positions 2 or 6; 2-AP, which has a 2-NH2 group but lacks a 6-NH2 group; c7A, in which the N7 group of adenine is replaced by CH; and hypoxanthine, which is the base of inosine and contains 6-keto and N1-H groups (Fig. 2). A comparison of the splicing efficiency of substrates that contain an adenine at the 3′-splice-site AG with those that contain a purine base (AG → PG) or c7A (AG → c7AG) may provide insights into the role of the 6-NH2 and N7 functional groups. Similarly, comparison of the splicing efficiency of substrates that contain a hypoxanthine (AG → HG) with those that contain a guanine (AG → GG) at the 3′-splice-site AG may provide insights into the contribution of the 2-NH2 group to catalytic step II. Finally, comparison of hypoxanthine (AG → HG) with purine (AG → PG) and guanine with 2-AP (AG → 2-APG) may provide insights into the contribution of the functional groups at positions 1 and 6 of adenine.
Comparison of the Effects of Replacing the 3′-Splice-Site Adenine with Cytosine, Uracil, or Guanine on Catalytic Step II.
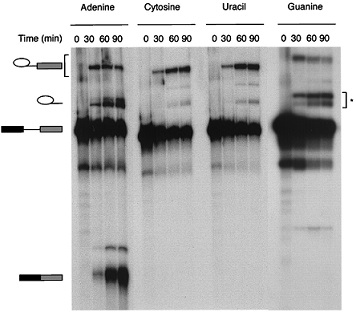
Previous studies have shown that replacement of the 3′-splice-site adenine with guanine (AG → GG) has an insignificant effect on the first step of the mammalian splicing reaction, but severely inhibits the second step (28–30). Here, we compare the effects of replacing the 3′-splice-site adenine with cytosine, guanine, and uracil. As shown in Fig. 3, all three base substitutions severely inhibit the second step of the splicing reaction. Quantitation of the data in Fig. 6 revealed that the guanine substitution reduced the efficiency of the second step by more than 100-fold. In contrast, cytosine and uracil substitutions resulted in an additional ≈10-fold reduction in second-step efficiency. This observation is consistent with previous studies in yeast showing that the second step of the splicing reaction is more severely affected by C or U substitutions than by G substitutions of the 3′-splice-site adenine (41, 42). We conclude that a purine-based nucleotide is preferred at the 3′-splice-site A position, and that the efficiency of the second step of splicing decreases in this order: guanine ≫ cytosine ≥ uracil.
The 6-NH2 and N7 Functional Groups of Adenine Are Not Required for Catalytic Step II.
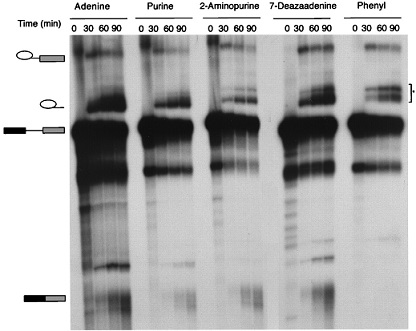
To examine the role of the 6-NH2 and N7 groups of the 3′-splice-site adenine, we determined the splicing efficiency of substrates that contain purine or c7A, respectively (for experimental details, see Materials and Methods). As shown in Fig. 4, neither the purine nor the c7A substitutions significantly decreased the efficiency of the second step of the splicing reaction (≈15% reduction with the purine substitution, and virtually no reduction with the c7A substitution). Interestingly, at the 90-min time point, the splicing efficiency of a pre-mRNA substrate bearing a c7A modification is slightly better than the wild-type (WT) substrate (Fig. 6). However, this slight increase in splicing efficiency could be an indirect effect, caused by the increased stability of the spliced mRNA that bears a modified base. We conclude that the 6-NH2 and N7 functional groups of adenine do not contribute significantly to the recognition of adenine during the second step of splicing.
Discussion
Several metazoan pre-mRNA sequence elements are involved in splice-site selection (4), including the GU dinucleotide at the 5′ splice site, the branchpoint sequence, and the pyrimidine tract/AG dinucleotide at the 3′ splice site. The RNA–RNA and RNA–protein interactions that occur at these sequences during spliceosome assembly appear to organize spatially the correct chemical groups for both steps of splicing (12–14). The AG dinucleotide, a sequence element that is highly conserved from yeast to humans, serves as a critical 3′-splice-site signal. A mutant pre-mRNA with an AG → GG substitution at the 3′ splice site can undergo the first step of splicing, but cannot generate significant amounts of spliced product (28–30). Thus, the AG dinucleotide is essential for exon ligation, and the chemical groups of adenine play a significant role in 3′-splice-site recognition. Furthermore, the presence of adenine as part of the 3′-splice-site signal in yeast, as well as the major and minor classes of mammalian introns and the self-splicing group II introns emphasize the important role of the 3′-splice-site adenine in RNA splicing. In addition, the AG dinucleotide at the 3′ splice site has been shown to be essential for the second-step reaction when the 3′ splice site is provided in trans (46).
In this study, we performed a systematic analysis of the functional group(s) in adenine that are required for the second step of the splicing reaction. The replacement of the 3′-splice-site adenine by a modified base does not significantly affect the first step of the splicing reaction, which indicates that spliceosome assembly and the first catalytic step are, for the most part, independent of AG recognition. Unlike the first step, the presence of a purine ring and its constituents as a 3′-splice-site signal is very important for the second-step chemistry; its importance is evident in the severe inhibition of the second step by the replacement of adenine with either cytosine or uracil (Fig. 3).
We have demonstrated that removal of either the 6-NH2 group (purine base) or the N7-nitrogen (c7A) has an insignificant effect on the efficiency of the second step of the splicing reaction (Fig. 4). The splicing efficiency of purine-substituted pre-mRNA decreased by ≈15%, and no effect was observed with the c7A substrate (Figs. 4 and 6). The introduction of a 2-NH2 group in the purine base (purine → 2-AP) resulted in a slight enhancement in splicing efficiency (see Fig. 4, and compare adenine with purine and 2-AP; also, see below). However, a guanine base, unlike 2-AP, has a 6-keto and a N1-H group (Fig. 2), and it resulted in severe inhibition of the second step (a >100-fold reduction; see Fig. 3). The 6-keto group in guanine, which is likely (in principle) to occupy the same position in the active site of the spliceosome as was occupied by the 6-NH2 group in adenine, is not expected to be responsible for this inhibition. The removal of the 6-NH2 group of adenine is not deleterious to the second step of splicing. Therefore, the chemical nature of the N1 position in the purine ring must be responsible for adenine recognition. Destabilization of the second-step active site caused by the 6-keto group in guanine is unlikely, but it cannot be ruled out.
Related articles And Qustion
See also
Lastest Price from Adenine manufacturers

US $0.00/kg2025-04-27
- CAS:
- 73-24-5
- Min. Order:
- 1kg
- Purity:
- 98.0%~102.0%; USP42
- Supply Ability:
- 30tons/month

US $0.00-0.00/kg2025-04-22
- CAS:
- 73-24-5
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 20MT