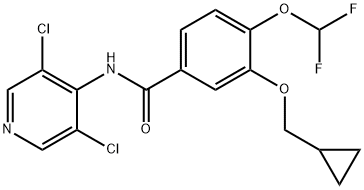
Roflumilast: Drug Pharmacology and Clinical Efficacy
Roflumilast is a selective phosphodiesterase-4 inhibitor indicated to decrease the risk of exacerbations in patients with severe chronic obstructive pulmonary disease (COPD) and to treat skin conditions such as plaque psoriasis and atopic dermatitis. It was first approved by the EMA in July 2010, and by the FDA in January 2018.
Pharmacology effects
Roflumilast is a highly selective phosphodiesterase-4 (PDE4) inhibitor. The resultant increase in intracellular cAMP induced by roflumilast's inhibition of PDE4 is thought to mediate its disease-modifying effects.
Roflumilast evidently has several pharmacological effects: antiinflammatory, anti-emphysema, and antibiotic actions. This drug also inhibits pulmonary hypertension and reduces mucus hypersecretion. The pharmacological actions leading to these effects are: a) inhibition of reactive oxygen species formation in epithelial cells, neutrophils and smooth muscle cells; b) inhibition of smooth muscle cell proliferation in the pulmonary artery, endothelial cells and probably some inflammatory cells causing pulmonary vascular remodeling; c) inhibition of fibroblasts, with a consequent reduction in pulmonary remodeling and, finally, d) inhibition of mucus production and improved ciliary beat frequency. In summary, roflumilast is the first non-steroidal anti-inflammatory drug that can be used in the treatment of COPD.
Clinical Efficacy
Roflumilast is a selective PDE4 inhibitor that inhibits pulmonary and systemic inflammation and rallies symptoms in airway diseases. Phosphodiesterase-4 inhibitors (PDE4) are of great interest for the treatment of airway inflammatory diseases due to its broad anti-inflammatory effects.
The newly discovered PDE4 inhibitor, roflumilast has exposed its potential in the treatment of Asthma, COPD and ACOS. Its mechanism of action in airway inflammatory diseases are said to be exerts by elevating intracellular cAMP and shows its anti-inflammatory action. Roflumilast reduced exacerbations and hospitalizations in our real-world population with severe COPD. AEs are common and frequently leading to discontinuation of roflumilast therapy.
References:
[1] AYKUT CILLI H G Halid Bal. Efficacy and safety profile of roflumilast in a real-world experience.[J]. Journal of thoracic disease, 2019, 11 4. DOI:10.21037/jtd.2019.04.49.[2] JULIO CORTIJO GIMENO E M S. Perfil farmacológico del roflumilast[J]. Archivos De Bronconeumologia, 2010, 46: 1-32. DOI:10.1016/S0300-2896(10)70052-1.
[3] XIAOLI ZHANG. Pharmacological mechanism of roflumilast in the treatment of asthma-COPD overlap.[J]. ACS Applied Energy Materials, 2018. DOI:10.2147/DDDT.S165161.
[4] SHRADHA BODKHE. Current insights on clinical efficacy of roflumilast for treatment of COPD, asthma and ACOS.[J]. ACS Applied Energy Materials, 2020. DOI:10.1016/j.intimp.2020.106906.
You may like
See also
Lastest Price from Roflumilast manufacturers

US $25.00/ASSAYS2025-04-21
- CAS:
- 162401-32-3
- Min. Order:
- 100ASSAYS
- Purity:
- 99.5%
- Supply Ability:
- 100 mt
US $1.00/kg2025-03-28
- CAS:
- 162401-32-3
- Min. Order:
- 1kg
- Purity:
- 99
- Supply Ability:
- 100