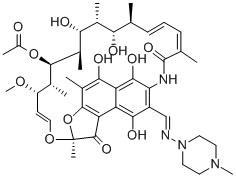
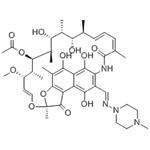
Rifampicin: Mechanism of action and Rifampicin resistance
Introduction of Rifampicin
Rifampicin is an important clinical antibiotic that belongs to the antimicrobial class of drugs. This drug is used for the management and treatment of various mycobacterial infections and gram-positive bacterial infections. It has antimicrobial activity against a wide range of gram-positive cocci, including Mycobacterium and Clostridium difficile, as well as specific gram-negative organisms, including Neisseria meningitidis, Neisseria gonorrhoeae and Haemophilus influenzae. Rifampicin is most commonly used as first-line treatment (in combination with other drugs) for mycobacteriosis, including tuberculosis, or for partially invasive staphylococcal infections (as part of combination therapy).

Mechanism of action
Rifampicin exerts its bactericidal and antimicrobial effects by inhibiting DNA-dependent RNA polymerase (RNAP). This inhibition occurs either by sterically obstructing the path of the elongating RNA at its 5' end or by reducing the RNAP's affinity for short RNA transcripts. Rifampin uniquely targets microbial RNAP, effectively arresting ongoing RNA synthesis. This effect is thought to be concentration dependent. Rifampin does not affect the mammalian RNAP enzyme, minimizing the range of potential adverse effects in humans.
Rifampicin resistance
Tuberculosis treatment requires months-long combination chemotherapy with multiple drugs, with shorter treatments leading to relapses. A major impediment to shortening treatment is that Mycobacterium tuberculosis becomes tolerant to the administered drugs, starting early after infection and within days of infecting macrophages. Multiple lines of evidence suggest that macrophage-induced drug tolerance is mediated by mycobacterial drug efflux pumps.
Using experiments that directly measure drug efflux, it was found that Mycobacterium tuberculosis transports the first-line antituberculosis drug rifampicin via a proton gradient-dependent mechanism. Verapamil, a known efflux pump inhibitor, inhibits macrophage-induced rifampicin tolerance and also inhibits rifampicin efflux from Mycobacterium tuberculosis.
As with macrophage-induced tolerance, the calcium channel-inhibiting property of verapamil is not required for its inhibition of rifampicin efflux. By testing verapamil analogs, we show that verapamil directly inhibits M. tuberculosis drug efflux pumps through its human P-glycoprotein (PGP)-like inhibitory activity. Screening commonly used drugs with incidental PGP inhibitory activity, we find many inhibit rifampicin efflux, including the proton pump inhibitors (PPIs) such as omeprazole. Like verapamil, the PPIs inhibit macrophage-induced rifampicin tolerance as well as intramacrophage growth, which has also been linked to mycobacterial efflux pump activity.
Rifampicin is not an inhibitor of tyrosinase
Rifampicin has been previously described as an inhibitor of tyrosinase. However, rifampicin contains a p-diphenol group and compounds with such a moiety have been shown before to reduce tyrosinase-generated o-quinones. Rifampicin also shows strong absorption in a region completely overlapping with the visible absorption band of dopachrome, the oxidation product of L-tyrosine and L-dopa, whose concentration is measured spectrophotometrically in the standard enzymatic assay to monitor the activity of tyrosinase. We have demonstrated that rifampicin is also rapidly oxidized by o-quinones generated from catechols by tyrosinase or by treatment with sodium periodate. Smaller changes of absorbance at 475 nm during oxidation of L-dopa by tyrosinase in the presence of rifampicin do not result from enzyme inhibition but from oxidation of rifampicin by dopaquinone, which leads to rapid decrease of rifampicin absorption in this range. The actual reaction rates are not affected, which we have demonstrated by measurements of oxygen consumption. Rifampicin behaves therefore as other compounds with reducing properties, such as ascorbic acid, hydroquinone, hydrazine derivatives, and flavonoids, some of which have also been incorrectly described before as inhibitors of tyrosinase.
References:
[1] M ALEXANDRA LAKE. The human proton pump inhibitors inhibit Mycobacterium tuberculosis rifampicin efflux and macrophage-induced rifampicin tolerance.[J]. Proceedings of the National Academy of Sciences of the United States of America, 2023. DOI:10.1073/pnas.2215512120.
[2] DAMIAN TARASEK; Hubert W. Rifampicin is not an inhibitor of tyrosinase[J]. International Journal of Biological Macromolecules, 2022. DOI:10.1016/j.ijbiomac.2022.07.217.
[3] PETRA SUDZINOVÁ. What the Hel: recent advances in understanding rifampicin resistance in bacteria.[J]. FEMS microbiology reviews, 2023. DOI:10.1093/femsre/fuac051.
You may like
Related articles And Qustion
See also
Lastest Price from Rifampicin manufacturers
US $0.00/kg2025-11-19
- CAS:
- 13292-46-1
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- Customise

US $0.00/kg2025-04-25
- CAS:
- 13292-46-1
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 1000kg