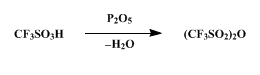
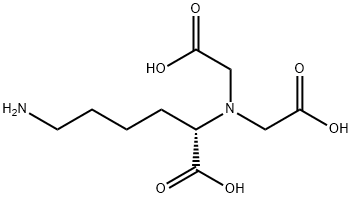
Reaction & Application on synthetic works of (S)-N-(5-Amino-1-Carboxypentyl)Iminodiacetic Acid Hydrate
(S)-N-(5-Amino-1-Carboxypentyl)Iminodiacetic Acid Hydrate(N,N-bis(carboxymethyl)- L-lysine, Nα.Nα-bis(methoxycarbonyl methyl)-L-lysine acid, Nα,Nα-bis (carboxymethyl)-L-lysine, Nα,Nα-bis(carboxymethyl)-L-lysine) is used as a metal chelating adsorbent for metal ion affinity chromatography. This method can be used for identification and rapid one-step purification of gene products expressed as fusion proteins with an oligo-histidine tag. The oligo-histidine tag serves as a high affinity binding sequence for the purification of fusion proteins via a metal chelating absorbent such as N-(5-Amino-1-carboxypentyl)iminodiacetic acid. Also soluble in DMSO:DMF 1:1 and DMSO:CH2Cl2 1:2
(S)-N-(5-Amino-1-Carboxypentyl)Iminodiacetic Acid Hydrate can also be used for preparation of radiopharmaceuticals such as radiotracter for disease diagnosis or radioactive therapeutic agent for disease treatment [1-4].
Example 1
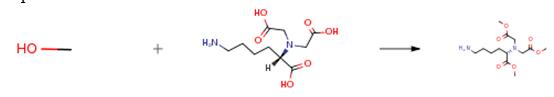
Hydrate of Na, Na-bis (carboxymethyl) -L-Lysine acid (0.93 g, 3.54 mmol) was added to 1.25 M hydrochloric acid (80 mL) dissolved in a methanol solution. The reaction time is preferably 24 hours. Concentration under reduced pressure gave the product 1.33 g (100 percent).
Example 2
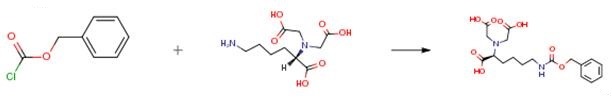
Dissolve Nα,Nα-Bis(carboxymethyl)-L-lysine hydrate (5 g, 0.019 mole) in saturated sodium bicarbonate aqueous solution (20 ml) and then is cooled in an ice bath. Take and dissolve benzyl chloroformate (3.4 ml, 0.0229 mole) in diethyl ether (20 ml) and then is slowly dropped into the above solution by an addition funnel. Then stir the solution at room temperature for 4 hours. Take aqueous phase of the solution and use diethyl ether for extraction (50 ml, 3 times). Take the aqueous phase and adjust the pH value to 2 by using concentrated hydrochloric acid, and cause some solid precipitated out. Filter and get the solid to obtain white solid product (5.9 g, 0.015 mole). The yield rate is 78 percent. Compound Data of the Product: 1H NMR (DMSO, 300 MHz): δ 7.36-7.20 (m, 5H), 4.98 (s, 2H), 3.41-3.20 (m, 5H), 2.92 (t, 2H), 1.58-1.24 (m, 6H). [0074] ESI-MS: m/z 397.19 (M+H)+ and 419.18 (M+Na)+.
Example 3
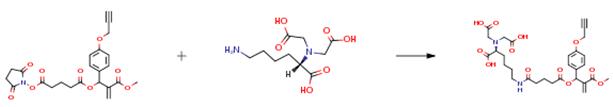
(Mono(1-(4-prop-2-ynyloxy-phenyl)-2-methoxycarbonyl-2-propenyl)glutaric acid 5S-carboxy-5-[bis(hydroxycarbonylmethyl)amino]pentylamide) is produced by the following procedure. To a mixture of NTA amine (131 mg, 0.5 mmol), and methanol (1 ml) was added triethylamine (101.2 mg, 0.14 ml, 1 mmol) to obtain a homogeneous solution, to which a solution of A-138B (5 ml, 0.1 M) in THF was added dropwise with stirring at 0° C. The mixture was allowed to warm to room temperature, and stirred overnight. It was then concentrated to dryness in vacuum. The residue was purified by flash chromatography (4:1 to 1:5 CHCl3/CH3OH) to yield the product (110 mg, 36percent) as a solid. 1H NMR (400 MHz, D2O): δ 1.21-1.70 (m, 6H), 1.86 (t, J=7.2 Hz, 3H), 2.18 (d, J=8.0 Hz, 2H), 2.44 (t, J=7.2 Hz, 2H), 2.92 (s, 1H), 3.00-3.20 (m, 6H), 3.70 (s, 3H), 4.78 (s, 2H), 5.98 (s, 1H), 6.45 (s, 1H), 6.55 (s, 1H), 7.04 (d, J=8.4 Hz, 2H), 7.39 (d, J=8.4 Hz, 2H). 13C NMR (100 MHz, D2O): δ 8.21, 20.56, 23.74, 26.59, 28.12, 33.04, 34.65, 38.89, 46.63, 52.45, 55.86, 68.85, 73.40, 76.78, 78.56, 115.07, 127.41, 129.15, 130.39, 138.32, 157.09, 167.13, 170.52, 174.04, 175.23.
References
1. Atomic Energy Council- Institute of Nuclear Energy Research (Thaiwan). Liu X; Zhang Y, Xu C. Compounds with double-functional group and method of manufacturing the same (by machine translation). TW2016/502[P], 2016, A, Paragraph 0019; 0034.
2. Atomic Energy Council - Institute of Nuclear Energy Research; Lin C, Chang Y, Wang J, Hsu C, Kuo W, Yu H, Lin W, Wang M. Method for Preparing Precursor Used for Labeling Hepatocyte Receptor and Containing Trisaccharide and Dtpa Ligand. US2014/46045[P], 2014, A1. Location in patent: Paragraph 0071-0074.
3. Clarkson University. Melman A. Method for selective derivatization of oligohistidine sequence of recombinant proteins. US9441010[P], 2016, B2. Page column 8.
4. Bolotin EM. Compositions for delivery of therapeutics and other materials, and methods of making and using the same. US2003/224974[P], 2003, A1. Page 23
You may like
Lastest Price from (S)-N-(5-AMINO-1-CARBOXYPENTYL)IMINODIACETIC ACID HYDRATE manufacturers

US $0.00/G2025-04-21
- CAS:
- 113231-05-3
- Min. Order:
- 10G
- Purity:
- 98%min
- Supply Ability:
- 300KG/month

US $30.00/kg2023-09-07
- CAS:
- 113231-05-3
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 20 tons
![330786-24-8 5-(4-phenoxyphenyl)-7H-pyrrolo[2,3-d]pyrimidin-4-ylamine; reaction; application; syntheses](https://www.chemicalbook.com/NewsImg/2019-11-6/20191161159448389.jpg)