Pyridinium Tribromide: Versatile Brominating Agent Driving Advances in Organic Synthesis
General Description
Pyridinium Tribromide (Py·Br3) is a versatile organic reagent widely used in organic chemistry and synthesis. Using Py·Br3 as a brominating reagent, 2,6,9-trisubstituted purines are brominated in high yields. This method is applicable to electron-rich purines with electron donors at the 2- and 6-positions. Pyridinium tribromide is a crystalline alternative to elemental bromine, and its use improves the bromination process of such substrates because the reagent is easy to handle and the work-up and purification procedures are simplified.

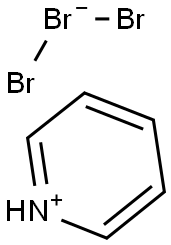
Figure 1. Pyridinium tribromide
Uses
Py·Br3 can be used as a catalyst and as a limiting reactant in the bromination of trans-cinnamic acid. It has been reported that the combination of potassium nitrate and Py·Br3 catalyzes the efficient oxidation of sulfides with air under transition metal-free conditions. The reaction system can also be used for the oxidation of alcohols by replacing Py·Br3 with bromine. In addition, novel pyrimidine derivatives can be prepared by a one-pot reaction of triethoxymethane, aromatic ketones and ammonium acetate using a nanomagnetic pyridine-tribromide (TBP-SCMNPs) catalytic system.
Chemoselective TBS deprotection of primary alcohols by Py·Br3 in MeOH
Catalyzed by a limited amount of Py·Br3 in MeOH, TBS primary alcohols can be chemoselectively deprotected at 0 °C in the presence of a variety of other protecting groups and common functional groups in moderate to excellent yields.
Safety
Py·Br3 is a hazardous chemical reagent that is volatile and highly corrosive. When Py·Br3 exists in rapid equilibrium with bromine in solution, it slowly releases bromine when dissolved. Contact or exposure to Py·Br3 is irritating to the skin and eyes. After ingestion, it may cause burns around the mouth and in the mouth. In vitro and in vivo studies have shown that humans exposed to pyridine may suffer damage to the liver, kidneys, and nervous system.1
References:
[1] DAVID BLIMAN . 8-Bromination of 2,6,9-trisubstituted purines with pyridinium tribromide[J]. Tetrahedron Letters, 2014, 55 18: 2887-2994. DOI:10.1016/j.tetlet.2014.03.084.[2] YU YUAN W L Xiao Shi. Transition-Metal-Free, ChemoselectiveAerobic Oxidations of Sulfides and Alcohols with PotassiumNitrate and Pyridinium Tribromide or Bromine[J]. Synlett, 2011, 4 1. DOI:10.1055/S-0030-1259516.
[3] AZIN KHARAZMI Dr S A Prof Ramin Ghorbani Vaghei. Synthesis of Pyrimidine Derivatives Catalyzed by Nanomagnetic Pyridinium-Tribromide Ionic Liquid[J]. ChemistrySelect, 2020, 5 4: 1247-1606. DOI:10.1002/slct.201904697.
[4] DIONICIO MARTINEZ-SOLORIO M P J. Chemoselective TBS deprotection of primary alcohols by means of pyridinium tribromide (Py·Br3) in MeOH[J]. Tetrahedron Letters, 2008, 49 35: 5117-5224. DOI:10.1016/j.tetlet.2008.06.072.
You may like
Lastest Price from Pyridinium tribromide manufacturers

US $0.00/kg2025-09-26
- CAS:
- 39416-48-3
- Min. Order:
- 1kg
- Purity:
- 92%
- Supply Ability:
- 1

US $10.00/KG2025-04-21
- CAS:
- 39416-48-3
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt