Pyflubumide: Synthesis and Introduction
Synthesis of Pyflubumide
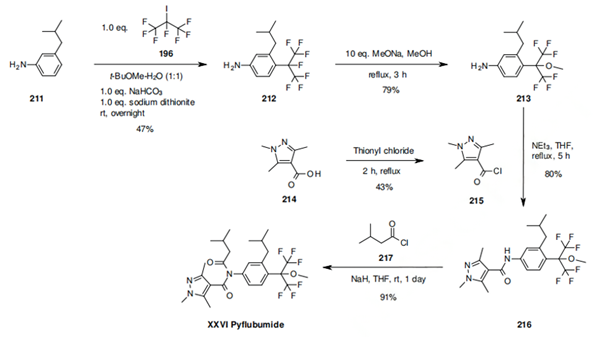
The synthesis from Nihon Nohyaku starts from 3-isobutylaniline (211), which is reacted with heptafluoroisopropyl iodide (196) via a radical reaction to generate compound 209. The fluoride in the 2-position of the isopropyl heptafluoridemoiety is displaced in a nucleophilic reaction by treatment with sodium methoxide in methanol. The aniline 213 is subsequently coupled with two acid chlorides as depicted in scheme 32 (initialy with the 1,3,5-trimethylpyrazole-4-carbonyl chloride 215 and then with isobutyl carbonyl chloride (217) to give pyflubumide (XXVI). The 1,3,5-trimethylpyrazole-4- carbonyl chloride 215 is prepared from the corresponding acid 214 with thionyl chloride at reflux for 2 hours.
Introduction of Pyflubumide
Pyflubumide is a novel acaricidal compound that has been developed by the Nihon Nohyaku Company. Pyflubumide is in fact a procide, with the active ingredient being the free N-H molecule which is obtained by in vivo cleavage of the acyl group. The metabolite (free N-H) 213 contains similar features to the carboxamide fungicides known to be succinate-dehydrogenase inhibitor. It was therefore postulated, and indeed verified, that the deacetylated form of pyflubumide (XXVI) is a mitochondrial complex II inhibitor. A double-inhibitor titration assay suggested that the metabolite of pyflubumide (213) may bind in a different mode at a different site than another known complex II acaricide, namely the metabolite of cyenopyrafen 210.