Clarithromycin: Properties, Action and use, ADME data etc.
Introduction
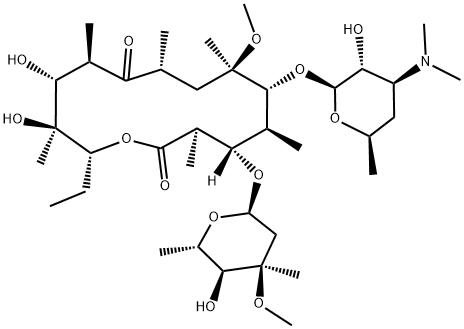
Clarithromycin (Figure 1) is a semisynthetic macrolide antibiotic, structurally related to erythromycin. It has a more favourable pharmacokinetic profile than erythromycin, thus allowing twice-daily administration and possibly increasing compliance among outpatients. Clarithromycin is well absorbed from the gastrointestinal tract and its systemic bioavailability (about 55%) is reduced because of first-pass metabolism. It undergoes rapid biodegradation to produce the microbiologically active 14-hydroxy-(R)-metabolite. The maximum serum concentrations of c1arithromycin and its 14-hydroxy metabolite, following single oral doses, are dose proportional and appear within 3 hours. With multiple doses, steady-state concentrations are attained after 5 doses and the maximal serum concentrations of c1arithromycin and of the 14-hydroxy derivative appear within 2 hours after the last dose. Clarithromycin is well distributed throughout the body and achieves higher concentrations in tissues than in the blood. Also, the 14-hydroxy metabolite exhibits high tissue concentrations, with values about one-third of the parent compound concentrations. The presence of food appears to have no clinically significant effect on c1arithromycin pharmacokinetics. The main metabolic pathways are oxidative N-demethylation and hydroxylation, which are saturable and result in nonlinear pharmacokinetics. The primary metabolite (14-hydroxy derivative) is mainly excreted in the urine with the parent compound. A reduction in urinary clearance in the elderly and in patients with renal impairment is associated with an increase in area under the plasma concentration-time curve, peak plasma concentrations and elimination half-life. Mild hepatic impairment does not significantly modify clarithromycin pharmacokinetics. [1]

Chemical and Physical Properties
Clarithromycin is a new semisynthetic macrolide derived from erythromycin A through a substitution of an O-methyl group at position 6 of the lactone ring. This chemical modification does not allow the hydrolysis of the lactone ring at low pH and prevents formation of the inactive 6,9-9,12 spiroketal derivative. Moreover, the molecule has better antimicrobial activity and pharmacokinetic properties in comparison with erythromycin. These characteristics allow a decrease in dosage and administration frequency and in the incidence of adverse effects affecting the gastrointestinal tract. Clarithromycin is a crystalline compound with a molecular weight of 748D, which is water insoluble and poorly soluble in alcohol, methanol and acetonitrile. The primary metabolite is the (R)-14-hydroxy epimer which also exhibits antimicrobial activity.[1]
Actions and Uses
Clarithromycin is a macrolide antibmtic structurally related to erythromycin with which it shares a broad spectrum of activity against common gram positive and gram negative pathogens, atypical organisms and some anaerobes. It is, however, more active than erythromycin against important respiratory pathogens, notably Haemophilus influenzae, Chlamydia pneumoniae and Legionella pneumophilla and more effective in atypical mycobacterial infections. This is attributed partly to the formation of an acnve 14-hydroxy metabolite, with which clarithromycin is synergistic, and to the high intracellular concentrations, which are achmved m the target tissues. Macrolide antibiotics are both bacteristatic and bactericidal. They inhibit protein biosynthesis essennal for growth and development after reversibly binding to a protein sub-unit of the bacterial ribosome. Resistance can develop if the cell wall blocks entry into the bacterial cytoplasm or if adaptation by the bacterium results in reduced binding to the ribosome. Bacterial strains developing resistance to erythromycIn are also resistant to Clarithromycin.[2]
ADME data [3]
See table 1 for main PK characteristics.

Other ADME data:
• Rat: PK of Clarithromycin in rats is superior to that of erythromycin, with 15-73 times higher concentrations in plasma and tissues; the peak level of Clarithromycin in lung was especially high.
• Human: PK of 14-OH metabolite is not linear with parent drug. Bioavailability is ~50% [FDA label]. Clarithromycin is 70% plasma bound. Steady-state peak plasma Clarithromycin concentrations of 1-2 μg/ml were reached in 2-3 hours with a 250-mg dose administered every 12 hours, and 3-4 mg/ml with a 500-mg dose administered every 8-12 hours. No significant differences in steady-state drug levels were seen with hepatic impaired or AIDS patients compared to healthy subjects. Extended-release tablets resulted in lower and later steady-state peak but equivalent AUCs [FDA label]. Good tissue penetration with 5 times more drug in lung compared with plasma and penetration into the middle ear [FDA label].
Human metabolic pathway: Clarithromycin is first metabolized to 14-OH Clarithromycin. The elimination half-life for Clarithromycin is 3-4 hours with 250 mg twice daily, and 5-7 hours with 500 mg 2-3 times daily. Clarithromycin is mainly excreted by liver and kidney, 30-40% excreted in urine depending on dose, an additional 10% excreted in urine as active metabolite, 14-OH Clarithromycin. It is not known if Clarithromycin is excreted into human milk [FDA label].
Safety and Tolerability
Animal toxicity: LD50 of Clarithromycin i.v. in mice was 184 mg/kg and 227 mg/kg in two separate studies. This was several times higher than the LD50 in rats (64 mg base/kg). These values were lower than those obtained following administration to mice by other routes. Signs of toxicity in both species were decreased activity, ataxia, jerks, tremors, dyspnea and convulsions.
Adverse effects were found on fetal development in monkeys, rats and mice; serum drug concentrations in the foetus are significantly higher than those in the mother [FDA label].
Hepatotoxicity occurred in all species tested (dog, rat, monkey): in rats and monkeys at doses 2 times greater than, and in dogs at doses comparable to, the maximum human daily dose [FDA label].
Renal tubular degeneration, testicular atrophy, corneal opacity and lymphoid depletion were all observed in animal testing [FDA label].
Human drug drug interactions: Clarithromycin inhibits cytochrome CYP3A4 and P-glycoprotein. Concomitant administration of Clarithromycin with cisapride, pimozide, or terfenadine is contraindicated due to cardiac arrhythmias (QT prolongation, ventricular tachycardia, ventricular fibrillation, and torsades de pointes) probably because of inhibition of hepatic metabolism of these drugs. Fatalities have been reported. Concomitant dosing of astemizole is not recommended for similar reasons and because of clinical experience with erythromycin [FDA label].
Human potential toxicity: Clarithromycin should not be used during pregnancy due to adverse effects seen in fetal development in monkeys, rats and mice.
Hepatotoxicity: increased liver enzymes, and hepatocellular and/or cholestatic hepatitis, with or without jaundice. Hepatic dysfunction may be severe but is usually reversible [FDA label].
Cardiac: QT prolongation and ventricular arrhythmias, including ventricular tachycardia and torsades de pointes, have been associated with clarithromycin [FDA label].
Hypoglycaemia occurs in rare cases [FDA label]. Human adverse reactions: Reactions are generally mild and the drug is well tolerated especially with slow-release tablets of Biaxin. In phase-1 clinical trials clarithromycin appears to be safe and well tolerated up to 1200 mg/day as single oral dose.
Adverse effects most commonly seen were gastrointestinal (diarrhoea, vomiting, abdominal pain and nausea), headache, and rash [FDA label].
In conclusion, Clarithromycin, because of its antibacterial activity and pharmacokinetic properties, appears to be a useful alternative to other macrolides in the treatment of community acquired infections.
References
[1] Fraschini F, Scaglione F, Demartini G. Clarithromycin clinical pharmacokinetics. Clin Pharmacokinet. 1993;25(3):189-204. doi:10.2165/00003088-199325030-00003
[2] MacConnachie AM. Clarithromycin (klaricid, Abbott). Intensive Crit Care Nurs. 1996;12(1):60-61. doi:10.1016/s0964-3397(96)81728-9
[3] Clarithromycin. Tuberculosis (Edinb). 2008;88(2):92-95. doi:10.1016/S1472-9792(08)70005-2
You may like
Lastest Price from Clarithromycin manufacturers

US $5.00-0.50/KG2025-05-07
- CAS:
- 81103-11-9
- Min. Order:
- 1KG
- Purity:
- 99% hplc
- Supply Ability:
- 500TONS

US $0.00-0.00/kg2025-04-28
- CAS:
- 81103-11-9
- Min. Order:
- 1kg
- Purity:
- 96.5%-102%;USP/EP
- Supply Ability:
- 5 tons