Eltrombopag: Synthesis and Mechanism
Introduction
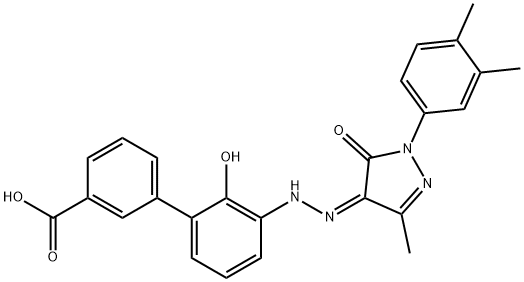
Eltrombopag (Figure 1), a non-peptide oral small molecule thrombopoietin (TPO) receptor agonist, is mainly used for the treatment of primary immune thrombocytopenia (ITP) clinically, especially thrombocytopenia in some patients with refractory anaphylactoid purpura who have been treated with glucocorticoids, immune proteins for injection, splenectomy or ineffective immunosuppressants. ITP is a condition that may cause unusual bruising or bleeding due to an abnormally low number of platelets in the blood. Eltrombopag has also been recently approved (late 2012) for the treatment of thrombocytopenia (low blood platelet counts) in patients with chronic hepatitis C to allow them to initiate and maintain interferon-based therapy. The advent of eltrombopag has brought new treatment options to the field of blood disorders.

Synthesis of eltrombopag
The synthetic route of eltrombopag has many steps, and the existing process route has multiple bottleneck steps, and has the problems of expensive raw materials, poor reaction selectivity in some steps, low reaction yield, and high production cost. Therefore, it is of great academic and economic value to explore a new synthesis process of eltrombopag that can be prepared on a large scale in line with the concept of green chemistry.
On the basis of literature investigation, the target product was synthesized from 2- nitrophenol by bromination, phenol hydroxy-methylation protection, Suzuki-Miyaura coupling, demethylation protection, nitro reduction and diazotization coupling. The feasibility of the designed route was confirmed by experimental study, and the reaction of each step has been improved and optimized, especially the following steps have achieved outstanding improvement:
(1) Optimization of preparation of 2-bromo-6-nitrophenol by bromination
Using 2-nitrophenol as raw material, toluene as solvent and cyclohexylamine as base, 2-bromo-6-nitrophenol was obtained by bromination reaction. The better process conditions obtained are: 2-nitrophenol: NBS (N-bromosuccinimide): cyclohexylamine is 1: 1: 1.1 (mole ratio), and the reaction is carried out at 5 °C for 6 hours, the yield is 77%. Compared with the literature, when the tert-butylamine in the literature is replaced by a new alkali, the post-reaction treatment process can be simplified and the industrial production cost can be reduced.
(2) Process optimization for the preparation of 2'-methoxy-3'-nitrobiphenyl-3- carboxylic acid by Suzuki-Miyaura coupling reaction
Using 2-bromo-6-nitroanisole as raw material, 3-carboxyphenylboric acid as boric acid reagent, sodium carbonate as base, dioxane and water as solvent, dichloro bis [ditert-butyl-(4-dimethylaminophenyl) phosphine] palladium (II) [Pd-132] as catalyst, 2- methoxy-3-nitrobiphenyl-3-carboxylic acid was obtained by Suzuki-Miyaura coupling reaction. The better process conditions obtained are: 2-bromo-6-nitroanisole: 3- carboxyphenylboronic acid: sodium carbonate: catalyst Pd-132 is 1:1.3:2:0.05 (molar ratio), and the reaction was carried out at 85 °C for 12 hours, the yield was 77%. Compared with the literature, when the new catalyst is used, the reaction time is greatly shortened, and the reaction yield is increased by 30%. In the intermediate synthesis process of eltrombopag, the use of the catalyst Pd-132 has not been reported in the literature.
(3) Study on the preparation of 3-methyl-1-(3,4-dimethylphenyl)-2-pyrazolin5-one by condensation Using 3,4-dimethylphenylhydrazine hydrochloride and ethyl acetoacetate as raw materials, ethanol as solvent, sodium methoxide as base, and sodium dithionite as metal sulfate reducing agent, 3-methyl-1-(3,4-dimethylphenyl)-2-pyrazolin-5-one was obtained by condensation reaction. The better process conditions obtained are: raw material 3,4-dimethylphenylhydrazine hydrochloride: ethyl acetoacetate: sodium methoxide is 1: 1.1: 1 (mole ratio), and the mass ratio of raw material and sodium hydrosulfite is 1: 0.1, after adding ethyl acetoacetate, the reaction temperature is 40℃ for two hours, then the temperature is 60 ℃ for 4 hours, the reaction yield is 87%.Compared with the literature, the novel organic base is improved, and a simple solvent washing method can be used for post-treatment, which reduces the difficulty of technological operation. Through the systematic study of the synthetic process of eltrombopag, the total yield reached 45%. Compared with the existing literature, the synthetic process obtained has the advantages of simple operation, low cost and easy availability of raw materials, and effective improvement of atomic economy. it provides a valuable research basis for the large-scale preparation of eltrombopag raw materials.[1]
Mechanism of action
Eltrombopag is an orally bioavailable, small-molecule TPO-receptor agonist that interacts with the transmembrane domain of the human TPO-receptor. Eltrombopag is a stimulator of STAT and JAK phosphorylation. Unlike recombinant TPO or romiplostim, Eltrombopag does not activate the AKT pathway in any way. It should be noted that when given to patients with aplastic anemia, other lineages besides platelet count were increased, suggesting that either eltrombopag enhanced the effect of TPO in vivo; or there is a yet uncovered mechanism of action at work.[2]
References
[1]Lu WY. Study on the Synthesis Process of Eltrombopag [D]. Zhengzhou University,2022.DOI:10.27466/d.cnki.gzzdu.2022.005088.
[2]Tarantino MD, Fogarty P, Mayer B, Vasey SY, Brainsky A: Efficacy of eltrombopag in management of bleeding symptoms associated with chronic immune thrombocytopenia. Blood Coagul Fibrinolysis. 2013 Apr;24(3):284-96. doi: 10.1097/MBC.0b013e32835fac99.
You may like
Lastest Price from Eltrombopag manufacturers

US $10.00/KG2025-04-21
- CAS:
- 496775-61-2
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $0.00/Kg/Bag2025-04-21
- CAS:
- 496775-61-2
- Min. Order:
- 2Kg/Bag
- Purity:
- 99% up, High Density
- Supply Ability:
- 20 tons