Pemetrexed Disodium: From Clinical Trials to Purity Control and Synthesis
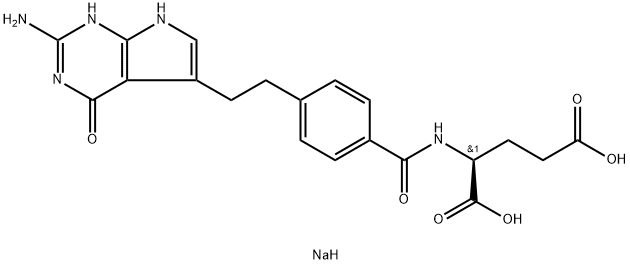
Pemetrexed disodium, a new multitargeted antifolate, is the first and only chemotherapy agent that has been granted marketing approval for use in combination with cisplatin (administered with vitamin B12 and folic acid) for the treatment of chemotherapy-naïve patients with unresectable malignant pleural mesothelioma. Also Pemetrexed disodium is a potent new antifolate which inhibits many folate-dependent reactions that are essential for cell proliferation. Its primary target is thymidylate synthase but it also inhibits folate-dependent enzymes involved in purine synthesis.

Phase 2 trial of pemetrexed disodium and gemcitabine
Gemcitabine has demonstrated single-agent activity in urothelial cancer with response rates that range from 24% to 28%. At the time this report was written, pemetrexed disodium was a new, novel, multitargeted antifolate compound that had demonstrated antitumor activity in the preclinical setting against a variety of solid tumors, including bladder cancers. The significant hematologic toxicity associated with the early use of this agent was evaluated extensively; and, subsequently, patients were supplemented with folic acid and vitamin B12, which led to a marked improvement in drug tolerance. Paz-Ares et al. conducted a phase 2 study of pemetrexed disodium in advanced transitional cell carcinoma of the bladder. Pemetrexed disodium was administered as a 10-minute infusion of 600 mg/m2 for the first 6 patients then 500 mg/m2 for all additional patients. In the preliminary report of 17 evaluable patients, 6 patients (35%) obtained a partial response (PR). It is noteworthy that the trial was performed without prophylactic vitamin supplementation, and there were 2 treatment-related deaths.[1]
Given the broad antitumor activity of both gemcitabine and pemetrexed disodium and their potential use in patients with compromised renal function, an evaluation of these agents given in combination was undertaken. Preclinical work demonstrated cytotoxic synergy when gemcitabine exposure preceded pemetrexed disodium in human colon cancer cells and bladder cancer in vitro. Adjei et al. performed a Phase I trial of the combination using a Day 1 and 8 schedule and demonstrated responses in a variety of epithelial cancers. On the basis of the demonstrated single-agent activity of these agents and the potential for developing an active nonplatinum-containing doublet, we conducted a phase 2 trial of combined gemcitabine and pemetrexed in patients with advanced urothelial cancer.
Doses of chemotherapy were based on actual weights, and body surface area was capped empirically at 2.2 m2. On Day 1 of therapy, pemetrexed disodium 500 mg/m2 was administered intravenously over 10 minutes followed 60 minutes later by gemcitabine 1000 mg/m2 intravenously over 30 minutes. The dose of gemcitabine was repeated on Day 8, and therapy was repeated every 3 weeks for a maximum of 6 cycles. The study was designed to assess the objective response rate and toxicity of the combination of pemetrexed disodium and gemcitabine. The primary endpoint was the proportion of patients responding to treatment, as measured using RECIST solid tumor response criteria. The trial was designed with the assumption that this doublet would be worthy of further study if the combination had a true response rate of 65%. Alternatively, a response rate of 45% would not be of interest.
Development of a purity control strategy for pemetrexed disodium
Pemetrexed is a synthetic compound used in the treatment of various cancers. The drug product is formulated as a lyophilized sterile powder of pemetrexed disodium that is reconstituted prior to intravenous administration. Key to assurance of patient safety and product quality is the development of a comprehensive understanding of the potential process and degradation impurities. In this paper we present stability indicating methodology for the determination of impurities in pemetrexed disodium drug substance (DS) and pemetrexed for injection drug product (DP). The drug substance method development for pemetrexed disodium focused on delivering a method that was robust and compatible with mass spectroscopy and charged aerosol detectors in order to provide control for the process and degradation impurities, impurity identity verification and response factor determination capability.[2]
Stability-indicating reversed phase HPLC methods have been developed and validated for the determination of 13 potential process and degradation impurities in pemetrexed disodium drug substance (DS) and pemetrexed for injection drug product (DP). This paper describes the development of HPLC-UV impurity methods for drug substance and drug product. Relative response factors (RRF) have been determined using HPLC-UV in tandem with CAD or by NMR detection. Conditions for the generation of system suitability solutions are described and assure adequate chromatographic resolution and peak identification without the need for impurity reference standards. The methods were fully validated and demonstrated to have acceptable specificity, linearity, accuracy, repeatability, intermediate precision, detection/quantitation limit, and robustness.
Preparation of Pemetrexed Disodium
Place 50 mmol of compound 1 prepared in Example 31 in a reactor, then add 200 mL of DMF, stir evenly at room temperature, then cool to 0°C,pass N2 for 15 minutes, add 110 mmol of N-methylmorpholine,65 mmol of 2-chloro-4,6-dimethoxy-1,3,5-triazine,after addition, move the reaction to room temperature and stir for 1 hour, then add 65 mmol of L-glutamic acid diethyl ester hydrochloride, continue stirring the reaction for 4 hours, TLC (dichloromethane: methanol = 6:1) monitors the reaction and adds 150 mL H2O and 150mL dichloromethane, stir evenly, stand and separate, add 120mL dichloromethane to the aqueous phase and extract twice, combine the organic phases, concentrate and remove the solvent, obtain brown oil, add 50mL methanol and 250mL ether to the oil, and then place in the refrigerator overnight, filter out the precipitated solid, wash the filter cake with a small amount of ether, obtain white solid. Add the obtained white solid to the reaction bottle, add 35mL 1N NaOH solution, stir at room temperature for 2h, monitor the reaction by TLC (dichloromethane: methanol = 6:1) until the reaction is complete. Add 40 mL of anhydrous ethanol to the reaction solution, stir for 10 minutes, adjust the pH to 7.5-8.5 with 1N hydrochloric acid, continue stirring for 30 minutes, heat the reaction solution to 60-65°C and keep the reaction warm for 0.5 hours, stop the reaction, stir the reaction solution and cool it to room temperature, then place it in a refrigerator overnight, filter it and obtain the crude product of pemetrexed disodium. The crude product was recrystallized with ethanol-water (3:1), dried to obtain an off-white pemetrexed disodium solid, with a yield of 65.5%.[3]
References
[1]Dreicer R, Li H, Cooney MM, Wilding G, Roth BJ; Eastern Cooperative Oncology Group. Phase 2 trial of pemetrexed disodium and gemcitabine in advanced urothelial cancer (E4802): a trial of the Eastern Cooperative Oncology Group. Cancer. 2008 Jun 15;112(12):2671-5.
[2]Warner A, Piraner I, Weimer H, White K. Development of a purity control strategy for pemetrexed disodium and validation of associated analytical methodology. J Pharm Biomed Anal. 2015 Feb;105:46-54.
[3]JIANGSU OCEAN UNIVERSITY - CN118619955, 2024, A
Lastest Price from Pemetrexed Disodium manufacturers

US $5.00-0.50/KG2025-05-08
- CAS:
- 150399-23-8
- Min. Order:
- 1KG
- Purity:
- 99% hplc
- Supply Ability:
- 500TONS

US $0.00/g/Bag2025-04-21
- CAS:
- 150399-23-8
- Min. Order:
- 1g
- Purity:
- 99%min
- Supply Ability:
- 10kg