Homopiperazine: synthesis and mainly application
Introduction
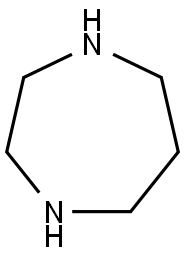
Homopiperazine (Figure 1) is nitrogen-containing heterocyclic rings containing symmetric nitrogen, which can be applied in medicine and chemical industries with strong biological activity and medicinal value after modification of nitrogen atoms.

Synthesis of homopiperazine
Most of the traditional synthesis methods require high temperature, high pressure, catalyst and hydrogen. Therefore, it is urgent to find a mild and low-cost synthetic route. In this paper, a synthetic route of homopiperazine was developed from ethylenediamine, which reacted with p-toluene sulfonyl chloride for amino protection, and then cyclized with 1,2-dichloroethane and 1,3-dichloropropane, respectively. The main research contents are as follows:
(1) In this paper, ethylenediamine was used as substrate in the synthesis of piperazine and homopiperazine. The efficient protection of amino groups on both sides of ethylenediamine has an important influence on the synthesis of target products, and it has important practical significance to establish the synthesis route. In the process of amino protection, p-toluene sulfonyl chloride was selected as the amino protection reagent, anhydrous sodium carbonate as the binding agent. The influence of the solvent type, the concentration of sodium carbonate and its dosage, the molar ratio of ethylenediamine to p-toluene sulfonyl chloride, reaction time, reaction temperature and other factors on the reaction were investigated, and the appropriate reaction conditions were obtained. Based on this, the optimized reaction conditions were studied by threefactor and three-level response surface, including molar ratio of ethylenediamine to ptoluene sulfonyl chloride, reaction temperature and reaction time. The optimum reaction conditions were obtained as follows: 0.02 mol ethylenediamine, 0.049 mol ptoluene sulfonyl chloride, 10 mL 20% sodium carbonate solution, 39 ℃, 3.9 h, and the yield of N,N'-bis(toluenesulfonyl)-1,2-ethylenediamine was 94.06 %.
(2) The cyclization of N,N'-bis(toluenesulfonyl)-1,2-ethylenediamine with 1,3- dichloropropane and the synthesis of homopiperazine from p-toluenesulfonyl were studied. The problems of insufficient reactant dissolution and long reaction time in the synthesis of N,N'-1,4-bis(4-methylphenyl)sulfonyl-homopiperzine were studied emphatically. Using N-methylpyrrolidone as solvent, the effects of molar ratio of N,N'- bis(toluenesulfonyl)-1,2-ethylenediamine to 1,3-dichloropropane, reaction time, reaction temperature, type of catalyst, amount of sodium hydroxide and volume of solvent on the reaction were investigated. After the optimization of response surface, the optimal cyclization reaction conditions were obtained, namely: N,N'- bis(toluenesulfonyl)-1,2-ethylenediamine 5 mmol, 1,3-dichlorohydrin 7.969 mmol, sodium hydroxide 0.548 g, solvent volume 25 mL at 123 ℃, the yield was 81.93% and the conversion was 100%. Secondly, the kinetic analysis of the synthesis reaction of N,N'-1,4-bis(4-methylphenyl)sulfonyl-homopiperzine was carried out at 115 ℃, 125 ℃ and 135 ℃ using the second-order reaction principle. The activation energy of the synthesis reaction of N,N'-1,4-bis(4-methylphenyl)sulfonyl-homopiperzine was 76.233kJ•mol-1 . The yield of piperazine was 87.02% under the same conditions of p-toluene sulfonyl group removal.[1]
Function and application
1. Homopiperazine Grafted Mesoporous Silicas fromRice Husk Ash for CO2 Adsorption
Chloro-functionalized mesoporous MCM-41, SBA-15, MCM-48 and KIT-6 were synthesized by cocondensation of 3-chloropropyl-trimethoxy-silane (CPTMS) and rice husk ash sodium silicate solution, which is subsequently grafted with a heterocyclic amine, homopiperazine (HPZ). X-ray powderdiffraction and BET analysis of the chloro-functionalized mesoporous silicas confirmed the similaritybetween their structural properties and those obtained from conventional silica sources. CO2 adsorption studies of all homopiperazine-grafted mesoporous silicas exhibited 8–10 wt% of adsorption capacity andare found to be selective, recyclable and thermally stable. Here, the CO2 adsorption reaction is viathe traditional carbamate mechanism. The presence of both secondary and tertiary amine in HPZinfluences the high CO2 adsorption capacity. Hence, these homopiperazine-grafted mesoporous silicas couldcontribute to CO2 capture as a green, tunable, selective and efficient sorbent.[2]
2.Homopiperazine derivatives as a novel class of proteasome inhibitors with a unique mode of proteasome binding.
The proteasome is a proteolytic machinery that executes the degradation of polyubiquitinated proteins to maintain cellular homeostasis. Proteasome inhibition is a unique and effective way to kill cancer cells because they are sensitive to proteotoxic stress. Indeed, the proteasome inhibitor bortezomib is now indispensable for the treatment of multiple myeloma and other intractable malignancies, but is associated with patient inconvenience due to intravenous injection and emerging drug resistance. To resolve these problems, we attempted to develop orally bioavailable proteasome inhibitors with distinct mechanisms of action and identified homopiperazine derivatives (HPDs) as promising candidates. Biochemical and crystallographic studies revealed that some homopiperazine derivatives inhibit all three catalytic subunits (ß 1, ß 2 and ß 5) of the proteasome by direct binding, whereas bortezomib and other proteasome inhibitors mainly act on the ß5 subunit. Proteasome-inhibitory homopiperazine derivatives exhibited cytotoxic effects on cell lines from various hematological malignancies including myeloma. Furthermore, K-7174, one of the homopiperazine derivatives, was able to inhibit the growth of bortezomib-resistant myeloma cells carrying a ß5-subunit mutation. Finally, K-7174 had additive effects with bortezomib on proteasome inhibition and apoptosis induction in myeloma cells. Taken together, homopiperazine derivatives could be a new class of proteasome inhibitors, which compensate for the weak points of conventional ones and overcome the resistance to bortezomib.[3]
3.Homopiperazine-rhodamine B adducts of triterpenoic acids are strong mitocans.
Parent pentacyclic triterpenoic acids such as ursolic-, oleanolic, glycyrrhetinic, betulinic and boswellic acid were converted into their acetylated piperazinyl amides that were coupled with rhodamine B. SRB assays to evaluate their cytotoxicity showed all of these triterpene-homopiperazinyl-rhodamine adducts 16-20 being highly cytotoxic for a panel of human tumor cell lines even in nanomolar concentrations while being significantly less cytotoxic for non-malignant cells. Interestingly enough, these compounds were even more cytotoxic than previously prepared piperazinyl analogs, thus making the homopiperazinyl spacer a very interesting scaffold for the development of biologically active compounds. Extra staining experiments showed that the cytostatic effect of compounds 18 and 20 onto A2780 cancer cells is due to their ability to act as a mitocan.[4]
References
[1]Ma JY. Study on synthesis route of piperazine and piperazine from ethylenediamine and process optimization [D].Zhengzhou University,2022.DOI:10.27466/d.cnki.gzzdu.2022.005242.
[2]Vinodh R, Bhagiyalakshmi M, Hemalatha P, et al. Homopiperazine grafted mesoporous silicas from rice husk ash for CO2 adsorption. J Nanosci Nanotechnol. 2014;14(6):4639-4648. doi:10.1166/jnn.2014.8240
[3]Kikuchi J, Shibayama N, Yamada S, et al. Homopiperazine derivatives as a novel class of proteasome inhibitors with a unique mode of proteasome binding. PLoS One. 2013;8(4):e60649. Published 2013 Apr 11. doi:10.1371/journal.pone.0060649
[4]Wolfram RK, Fischer L, Kluge R, Ströhl D, Al-Harrasi A, Csuk R. Homopiperazine-rhodamine B adducts of triterpenoic acids are strong mitocans. Eur J Med Chem. 2018;155:869-879. doi:10.1016/j.ejmech.2018.06.051
See also
Lastest Price from Homopiperazine manufacturers
US $0.00-0.00/KG2025-11-12
- CAS:
- 505-66-8
- Min. Order:
- 1KG
- Purity:
- 98
- Supply Ability:
- 10000KGS
US $0.00-0.00/KG2025-04-04
- CAS:
- 505-66-8
- Min. Order:
- 1KG
- Purity:
- 98%
- Supply Ability:
- 1ton