Prussian Blue: Both a Dye and a Medication
Basic Introduction
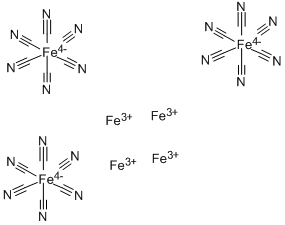
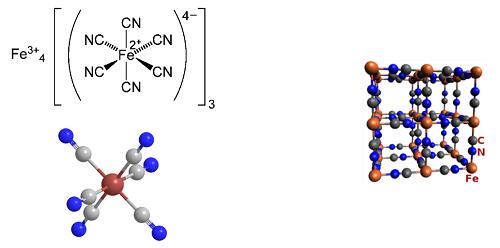
The pigment Prussian blue consists of iron cations, cyanide anions, and water. The empirical formula—minus the water of crystallization—is Fe7(CN)18. This seems odd with respect to the iron oxidation state until you learn that the complex contains Fe(II) and Fe(III). Thus, the formula that gives a truer idea of its composition is Fe4[Fe(CN)6]3. Its formal name is iron(III) hexacyanoferrate(II). As shown in the two left-hand drawings, the Fe(CN)6 anion in Prussian blue is octahedral. The right-hand drawing shows its unit cell, which has a cubic lattice structure. In addition to having as many as 16 molecules of water per formula unit, the compound usually contains inorganic impurities, which can affect its color.[1]
The name Prussian blue originated in the 18th century, when the compound was used to dye the uniform coats for the Prussian army. Over the years, the pigment acquired several other "blue" names, including Berlin, Parisian, and Turnbull’s blue. It has been used for centuries in unusually diverse applications (see the information box). Despite the presence of cyanide groups, the pigment is not toxic to humans. Seniors among us will recognize “Prussian blue” as a crayon color. Prussian blue was one of the 38 original Crayola colors introduced by Binney & Smith Inc. in 1903. (The company name was subsequently changed to Crayola; later, the firm was acquired by Hallmark.) The Prussian blue crayon name lasted until 1958, when it was changed to midnight blue. The reason for the change is unclear: One source says it was made because no one knew what Prussia was anymore; another reports that the move was spurred by political correctness during the Cold War.
Uses for Prussian Blue
Paints, inks, and enamels
Prussian Blue is a deep and inky colour. Its influence can be seen across art history– it is the characteristic pigment of Picasso’s Blue Period, it was a favourite among the painters of the Baroque and Rococo periods, and it is the colour that is produced in the cyanotype process (thus giving us the term ‘blueprint’). This article looks at the intriguing history of the colour, its use in Japanese printmaking, and why acrylic painters are hard-pressed to find a genuine Prussian Blue paint. Prussian Blue was created by accident in 1704. There are varying accounts as to the exact story behind the colour, but the most interesting is from German physician Georg Ernst Stahl (1659–1734). He says that a pigment maker in Berlin named Diesbach was making a batch of a Red Lake pigment using potash, crushed cochineal insects, and iron sulfate. Having run out of potash, he went to local pharmacist Johann Konrad Dippel to purchase some more. Dippel, possibly looking to fleece Diesbach, sold him potash that was contaminated with dried cattle blood. When Diesbach tried to make his red pigment, it made a deep blue pigment instead. Dippel knew that it was his adulterated potash that had caused this reaction and saw a business opportunity, so he conducted further experiments and commercialised the colour under the name Berlin Blue. Dippel kept the composition of the pigment a close secret, thus amassing a considerable fortune. Unfortunately for Dippel, an English chemist reverse-engineered it in 1724 and published the formulation. With his source of income all but gone, Dippel left Berlin, but his enterprising didn't stop there. He claimed he had discovered an elixir of life and tried to buy a castle in exchange for it (an offer that was rejected). Rumours spread accusing him of grave-robbing, experimenting with cadavers, and being in league with the devil. Some even theorise that he inspired Mary Shelley's Frankenstein.[2]

Putting aside its rather intriguing origin story, Prussian Blue was an extremely important pigment development. It was the first synthetic blue pigment to be invented since the ancient Egyptians invented Egyptian Blue. The next big developments– Cobalt Blue and synthetic Ultramarine Blue– came more than a century later. While Prussian Blue was enthusiastically adopted by European easel painters, it was Japanese artists who arguably made best use of its tonal capabilities. It took centre-stage in 19th century Japan through the aizuri-e style of woodblock printing, in which the image is printed predominantly in blue. Aizuri-e had traditionally used indigo ink, but Prussian Blue was found to be more lightfast and more rich in colour. Its particular suitability for this mono-pigment technique hinges on its capability of a range of tonal values, and we can find this in the work of many Japanese artists of the Edo period. The most famous in Europe is undoubtedly Katsushika Hokusai (1760-1849) His series 36 Views of Fuji are made almost entirely with Prussian Blue ink. As exemplified in the above print, it could be applied in thin, transparent layers to the block for graduating washes of light blue that give an impression of looking into the hazy distance, but it was equally adept for dark passages of colour that are near black, like we see on the tip of Mount Fuji and the figures in the foreground. Even just using one colour, it gives an overall impression of depth and a full tonal range.
Radiation Medications
There are multiple radioactive elements in the world, but Prussian blue effectively deals with two of them: cesium and thallium. If a patient has been exposed to dangerous levels of either of these elements, the liver will filter them out of the body only for them to be reabsorbed again once they enter the GI tract. This process will be repeated until the substances decay. This is an issue because of the 'biological half-life' – the amount of time it takes half of the radiation to disappear. Cesium and thallium have a biological half-life of 110 days and three days, respectively. A hundred and ten days is a long time to have a big chunk of radiation floating around in your body, and if something isn't done about this as soon as possible, it can very easily lead to radiation poisoning. But this is where Prussian blue comes in. It can effectively cut the biological half-life of cesium down to 30 days, while thallium is shortened to 3 days, a significant time reduction that can save many lives. Prussian blue can bind to the radioactive elements and keep them from being reabsorbed within the intestines. The radiation is then passed out of the body in the stool. Because of the rapid elimination of this radiation, there is less time for it to cause damage inside the body. The patient ends up with blue, radioactive poop for as long as they take the medication, but they also have a decreased risk of cancer and dying from radiation poisoning. It must be remembered that this is all that Prussian blue does. It helps to flush radioactive cesium and thallium out of the body. It won't treat the signs and symptoms of radiation illness, reverse the effects of gamma-ray exposure, or remove anything other than cesium and thallium. This isn't a wonder drug that fixes all radiation problems. But it does help to address the root cause of some of them, and that's something to be thankful for.[3]
From all the research that this author is aware of, it does appear that Prussian blue is a safe and effective means of treating some types of radiation poisoning. This is something that's been around for a long time, and though the events where it needs to be used are relatively rare, we don't see people growing a third eye in the middle of their forehead or turning purple after they take it or anything like that. To be most effective, Prussian blue should be administered as soon after the radiation exposure as possible. Since it is only available via prescription, seeking professional medical care as quickly as possible is paramount. Typically, the dosage is a 500mg capsule, three times a day for 30 days, with the main side effects being constipation and severe stomach pain. Seeing that people who take this will expel radioactive material from their bodies, it's recommended that they flush the toilet three times with the lid down after each successful bowel movement. The drug has received FDA approval for treating radiation poisoning, and the CDC says it is even safe for children 2-12 years old and pregnant women. There probably needs to be a bit more research here, however, to determine how it is safe for a pregnant woman (with a baby inside of her), but we're not sure if it's safe for a newborn-two-year-old. We're also not sure if Prussian Blue can pass through breastmilk. However, this seems to be a reasonably safe drug for everyone else. And history backs this statement up.
References
[1] Concina, I. (2024). An Old Material for a New World: Prussian Blue and Its Analogues as Catalysts for Modern Needs. Inorganics, 12(4), 124
[2] Claire Gervais,Marie-Angélique Languille,Solenn Reguer,Chantal Garnier & Martine Gillet.(2014).Light and anoxia fading of Prussian blue dyed textiles.Heritage Science(1),1-8.
[3] He, H., Wang, Y., Zhang, Y., et al. The Application of Prussian Blue Nanoparticles in Tumor Diagnosis and Treatment. Sensors 2020, 20(12), 3589
See also
Lastest Price from Prussian Blue manufacturers

US $65.00-20.00/kg2025-06-25
- CAS:
- 14038-43-8
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 20MT

US $100.00/kg2025-04-21
- CAS:
- 14038-43-8
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10 mt