Propylparaben: A Comprehensive Overview for Chemical Professionals
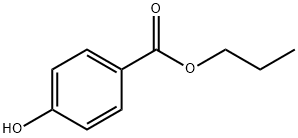
Propylparaben is the n-propyl ester of p-hydroxybenzoic acid. It occurs as a natural substance found in many plants and some insects. Additionally, it can be manufactured synthetically for use in cosmetics, pharmaceuticals, and foods.

Synthesis
One of the simplest ways to produce propylparaben is through the esterification of 4-hydroxy benzoic acid with propanol using an acidic catalyst.The first major step includes the protonation of the carbonyl due to the acidic conditions. This protonation results in a positive charge on the carbonyl which will offset the electron density from the ester carbon atom, this allows the propanol to preform a nucleophilic attack on the carbonyl.The proton of the nucleophilic propanol is then transferred by the solvent to the esters hydroxyl group. The hydroxyl can then act as a good leaving group and be expelled from the tetrahedral intermediate as water, allowing the ester carbonyl group to reform. Finally, deprotonation of the reformed carbonyl group will produce the final ester product, propylparaben.1
Main Components
Propylparaben is characterized by its chemical structure consisting of a benzene ring substituted with a hydroxyl group (OH) and an ester group linking to a propyl chain. The presence of the hydroxyl group enhances the hydrophilicity of the compound, while the propyl group balances its lipophilicity, making it effective in a variety of mediums.
Uses
Parabens are used for their antimicrobial properties and are found in a range of products, with methylparaben and propylparaben, frequently found as preservatives in food, cosmetics, PCPs, and drugs.
Precautions
In a recent study, La Merrill and co-workers showed that chronic exposure to parabens increased breast cancer tumor volume and metastasis to the lungs in a mouse model (Tong et al., 2023). In the accompanying RNA-seq experiments, they found that methylparaben and propylparaben differentially regulated 288 and 412 genes, respectively, with 158 overlapping both treatments. Interestingly, pathway analysis failed to find enrichment of estrogen signaling, but subsets of genes associated with human breast cancer and metastasis were identified (Tong et al., 2023).2
References
1.Hazarika, Mridul; Parajuli, Raghab; Phukan, Prodeep (January 2007). "Synthesis of parabens using montmorillonite K10 clay as catalyst: A green protocol" (PDF). Indian Journal of Chemical Technology. 14: 104–106.
2.Raj Kumar, Iain J. McEwan,Chapter 6 - Endocrine disruptors: the enemy without,Editor(s): Raj Kumar, Iain J. McEwan,Steroid Hormone Receptors in Health and Disease,Academic Press,2024,Pages 107-123.
You may like
Related articles And Qustion
Lastest Price from Propylparaben manufacturers

US $0.00-0.00/kg2025-12-11
- CAS:
- 94-13-3
- Min. Order:
- 1kg
- Purity:
- 98.8%
- Supply Ability:
- 1000kg
US $0.00-0.00/kg2025-11-28
- CAS:
- 94-13-3
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1000kg