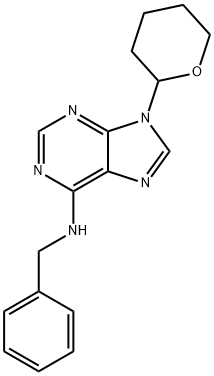
N-Benzyl-9-(tetrahydro-2H-pyran-2-yl)adenine: An Emerging Compound in Medicinal Chemistry
Introduction
In the dynamic field of medicinal chemistry, the synthesis of new compounds is pivotal for discovering novel therapeutic agents. N-Benzyl-9-(tetrahydro-2H-pyran-2-yl)adenine has recently emerged as a compound of significant interest. This synthetic adenine derivative, incorporating a unique tetrahydro-2H-pyran ring, represents a noteworthy advance in the modification of nucleobase analogs, aimed at enhancing biological activity and pharmacokinetic properties[1].
Synthesis
The synthesis of N-Benzyl-9-(tetrahydro-2H-pyran-2-yl)adenine is a fascinating example of modern organic chemistry's capabilities in crafting complex molecules. The process begins with the selection of adenine as the nucleobase foundation, which is then modified at the N9 position. This modification involves the introduction of a benzyl group, which serves not only as a protective group but also enhances the overall molecular stability and reactivity necessary for further reactions.
Following the initial modification, the critical step involves the Michael addition where the adenine derivative reacts with a pre-formed activated alkene derived from tetrahydropyran. This step is meticulously optimized to ensure regioselectivity and the correct formation of the tetrahydro-2H-pyran ring, which is pivotal for the desired biological activities. The synthesis concludes with careful purification techniques, ensuring the final product is free from impurities and ready for biological evaluation.
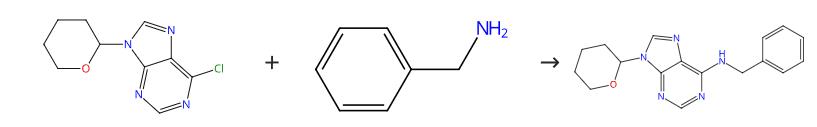
Figure 1: Synthesis pathway of N-Benzyl-9- (tetrahydro-2H-pyran-2-yl) adenine
Applications
The potential applications of N-Benzyl-9-(tetrahydro-2H-pyran-2-yl)adenine are broad and impactful. Primarily, this compound is being investigated for its antiviral and anticancer properties. Its unique structure may interfere with DNA and RNA processes, making it a candidate for targeting viral replication mechanisms or cancer cell proliferation. Moreover, researchers are exploring its use in neuroprotective treatments, given adenine derivatives' potential role in cellular metabolism and protection against neurodegenerative diseases. Additionally, the compound's ability to modulate enzymatic activities involved in cellular repair and apoptosis suggests potential uses in managing aging-related conditions and enhancing chemotherapy efficacy. These diverse applications underscore its versatility and the promise it holds in contributing to multiple therapeutic areas[2].
Pharmacokinetic Characteristics
The pharmacokinetic profile of N-Benzyl-9-(tetrahydro-2H-pyran-2-yl)adenine is crucial in determining its suitability as a drug. Early studies indicate that the tetrahydro-2H-pyran ring may enhance the molecule's solubility and facilitate easier crossing of cellular membranes, thus improving its bioavailability. This alteration could also impact the distribution of the drug within the body, targeting tissues more effectively and potentially reducing the frequency of dosing required. Furthermore, the modification may lead to a more favorable metabolism, where the drug exhibits a slower rate of degradation, thus maintaining therapeutic levels in the bloodstream for longer periods. Such characteristics are advantageous in developing medications that require less frequent administration, improving patient compliance and overall treatment efficacy. These preliminary findings highlight the importance of continuing in-depth pharmacokinetic evaluations to fully understand and optimize the drug's performance in human physiology.
Toxicity
A comprehensive understanding of the toxicity of N-Benzyl-9-(tetrahydro-2H-pyran-2-yl)adenine is essential for its development as a safe drug. Initial toxicological assessments focus on determining its cytotoxic levels, interaction with major organ systems, and potential genotoxic effects. These studies are crucial for mapping out the therapeutic window of the compound and ensuring that it can be administered safely to patients. Additionally, assessments include evaluating potential immunotoxic effects, which could impact patient safety and efficacy when administered over long periods. These insights are vital for advancing clinical trials.
Conclusion
N-Benzyl-9-(tetrahydro-2H-pyran-2-yl)adenine stands out as a compelling new player in the field of medicinal chemistry. Its innovative structure and promising early pharmacological data suggest it could lead to new treatments for several serious diseases. Continued research will be vital in fully unraveling its mechanisms of action, pharmacokinetics, and safety profile. For professionals in the chemical and pharmaceutical industries, developments like these underscore the importance of continuous innovation and exploration in the quest for better therapeutic solutions. The journey of N-Benzyl-9-(tetrahydro-2H-pyran-2-yl)adenine from the lab to potentially becoming a part of the medical arsenal illustrates the exciting and complex nature of drug development.
References
[1]Nyman L P, Gonzales C J, Arditti J. Reversible structural changes associated with callus formation and plantlet development from aseptically cultured shoots of taro (Colocasia esculenta var antiquorum)[J]. Annals of Botany, 1983, 51(3): 279-286.
[2]Jackson G V H, Ball E A, Arditti J. Tissue culture of taro, Colocasia esculenta (L.) Schott[J]. Journal of Horticultural Science, 1977, 52(3): 373-382.
You may like
Related articles And Qustion
See also
Lastest Price from N-Benzyl-9-(tetrahydro-2H-pyran-2-yl)adenine manufacturers
US $0.00/kg2025-03-03
- CAS:
- 2312-73-4
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10000KGS