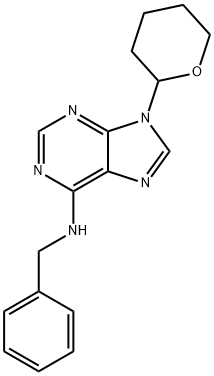
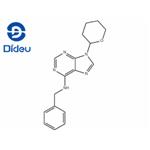
N-Benzyl-9-(tetrahydro-2H-pyran-2-yl)adenine: Synthesis and Applications in Chemistry and Plants Growth
General Description
N-Benzyl-9-(tetrahydro-2H-pyran-2-yl)adenine is a highly mobile synthetic cytokinin. Foliar spray of N-Benzyl-9-(tetrahydro-2H-pyran-2-yl)adenine increased branching in carnation, chrysan-themum, pointsettia, petunia and fuchsia. Application of N-Benzyl-9-(tetrahydro-2H-pyran-2-yl)adenine to flower buds at an early stage increased both the diameter and the fresh weight of carnation flowers or chrysanthemum infloresences. In Lilium longiflorum, spraying with N-Benzyl-9-(tetrahydro-2H-pyran-2-yl)adenine resulted in delayed anthesis and increased dry matter accumulation in flowers under high photosynthetic photon flux. Application of N-Benzyl-9-(tetrahydro-2H-pyran-2-yl)adenine induced the formation of numerous bulbils in the leaf axils.

Figure 1. Properties of N-Benzyl-9-(tetrahydro-2H-pyran-2-yl)adenine
Preparation
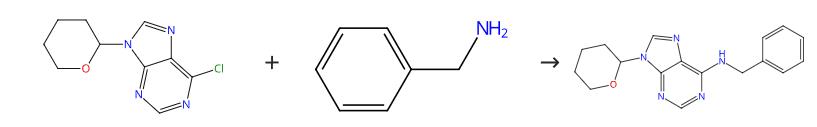
Figure 1. Preparation of N-Benzyl-9-(tetrahydro-2H-pyran-2-yl)adenine
Dissolve alcohol in PrOH (14 ml). Add TEA (0.95 ml, 6.9 mmol) and 6-chloro-9-(tetrahydropyran-2-yl)purine (1.64 g) to the reaction mixture. Heat the reaction mixture at 45 °C for 6 hours. Cool the reaction mixture. Filter the mixture. Evaporate the filtrate partly. Purify the filtrate by CC (60.3 g of silica gel, mobile phase CHCl3-Me2CO 4:1, flow rate 7.5 ml/min). The product is a light yellow viscous substance that starts to crystallize after two weeks of drying over P2O5 in a vacuum desiccator.1
Applications
Intermediate in Cosmetics Synthesis
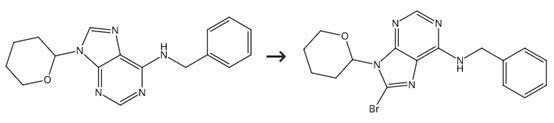
Figure 2. Intermediate in Cosmetics Synthesis
Dissolve N-Benzyl-9-(tetrahydro-2H-pyran-2-yl)adenine (2.14 mmol) under argon in dry THF (1 mmol/6.9 ml). Treat the solution dropwise with LDA solution during 15-60 minutes (21 ml, 42 mmol). Stir the reaction mixture at 78 °C for 1-1.5 hours. Add a solution of CBr4 (4.58 mmol) in dry THF (13.8 ml) dropwise to the reaction mixture during 12-35 minutes. Stir the reaction mixture for 1 hour. Add 20% NH4Cl (44 ml) dropwise to the reaction mixture. Warm the reaction mixture spontaneously to room temperature. Separate the mixture into different layers. Extract the aqueous layer. Dry the collected organic layers with Na2SO4. Evaporate the collected organic layers. Purify the crude product by CC-mobile phase: hexane-EtOAc (3:2).2
Growth-promoting Factors in Plants
In a study reported in 1967, N-Benzyl-9-(tetrahydro-2H-pyran-2-yl)adenine was prepared and found to promote chlorophyll retention (and senescence delay) in plant tissues exceptionally strongly, and growth of tobacco callus almost as strongly as 6-benzylaminopurine(BAP). Its high activity was attributed to the lability of the 9-substituent. Other early studies showed that some synthetic N-Benzyl-9-(tetrahydro-2H-pyran-2-yl)adenine derivatives could stimulate tiller bud elongation in cereals and increase numbers of apple and grape fruits. A comparative study published in 1981 demonstrated that activities of BAP and various 9-substituted derivatives in the promotion of lettuce seed germination declined in the following order: BAP = 9-N-Benzyl-9-(tetrahydro-2H-pyran-2-yl)adenine > 9-methyl BAP > 9-methoxymethyl BAP > 9-cyclopentyl BAP > 9-cyclohexyl BAP. Later, 6-benzylamino-9-(tetrahydrofuran-2-yl)purine (BAP9THF) was prepared, its impact on leaf senescence was studied, and both N-Benzyl-9-(tetrahydro-2H-pyran-2-yl)adenine and BAP9THF were found to delay senescence and induce several growth responses more strongly than BAP. The increased senescence-retarding activity of these compounds was at least partially attributed to the gradual cleavage of pyranyl or furanyl and release of free base there form. 6-benzylaminopurine and N-Benzyl-9-(tetrahydro-2H-pyran-2-yl)adenine have been reported to induce adventitious shoot formation significantly more strongly than iP or Kin. Generally, 2-tetrahydropyranyl and 2-tetrahydrofuranyl cyclic ether groups are widely used in organic chemistry as protective groups and can be easily removed in acidic conditions. The 9THP- or 9THF-substituted Kin and other 9THP and 9THF ArCKs have significant anti-senescence effects, as previously described for BAP.3 In attempt to improve specific biological properties of CKs reported in 2009, a number of new hydroxyl and/or methoxy benzene ring-substituted 9THP and 9THF cytokins were synthesized and tested. They were prepared via the condensation of 6-chloropurine with 3,4-dihydro-2H-pyran or 2,3-dihydrofuran, catalyzed by trifluoroacetic acid, followed by coupling of the intermediates with corresponding benzylamines. The 9THP and 9THF ArCKs were found to have higher activities than corresponding free bases in tobacco callus, WLS, and Amaranthus bioassays. Not all the prepared 9THP and 9THF derivatives are entirely stable at pH < 4, because they slowly decompose to their free bases.3
References
1. Phytochemistry (Elsevier) (2017), 135, 115-127.
2. WO2016095880 A1 2016-06-23.
3. Vylí?ilová H, Bryksová M, Matu?ková V, Dole?al K, Plíhalová L, Strnad M. Naturally Occurring and Artificial N9-Cytokinin Conjugates: From Synthesis to Biological Activity and Back. Biomolecules. 2020 May 29;10(6):832. doi: 10.3390/biom10060832.
You may like
Related articles And Qustion
Lastest Price from N-Benzyl-9-(tetrahydro-2H-pyran-2-yl)adenine manufacturers
US $0.00/kg2025-03-03
- CAS:
- 2312-73-4
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10000KGS